TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy
- PMID: 29037207
- PMCID: PMC5644120
- DOI: 10.1186/s13024-017-0216-6
TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy
Abstract
Background: Genetic variants of the Triggering Receptor Expressed on Myeloid Cells-2 (TREM2) confer increased risk of developing late-onset Alzheimer's Disease (LOAD) and other neurodegenerative disorders. Recent studies provided insight into the multifaceted roles of TREM2 in regulating extracellular β-amyloid (Aβ) pathology, myeloid cell accumulation, and inflammation observed in AD, yet little is known regarding the role of TREM2 in regulating intracellular microtubule associated protein tau (MAPT; tau) pathology in neurodegenerative diseases and in AD, in particular.
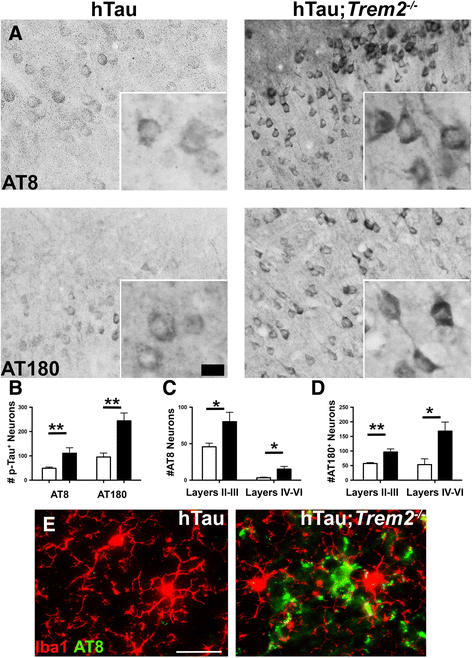
Results: Here we report that TREM2 deficiency leads to accelerated and exacerbated hyperphosphorylation and aggregation of tau in a humanized mouse model of tauopathy. TREM2 deficiency also results, indirectly, in dramatic widespread dysregulation of neuronal stress kinase pathways.
Conclusions: Our results suggest that deficiency of microglial TREM2 leads to heightened tau pathology coupled with widespread increases in activated neuronal stress kinases. These findings offer new insight into the complex, multiple roles of TREM2 in regulating Aβ and tau pathologies.
Keywords: Alzheimers disease; Immunity; Inflammation; TREM2; Tauopathy.
Conflict of interest statement
Ethics approval
All experimental design and analysis performed was overseen and approved by the Cleveland Clinic Lerner Research Institute Institutional Review Board (IRB), as well as the Cleveland Clinic Institutional Animal Care and Use Committee (IACUC).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

