The efficacy and stability of an information and communication technology-based centralized monitoring system of adherence to immunosuppressive medication in kidney transplant recipients: study protocol for a randomized controlled trial
- PMID: 29037222
- PMCID: PMC5644178
- DOI: 10.1186/s13063-017-2221-z
The efficacy and stability of an information and communication technology-based centralized monitoring system of adherence to immunosuppressive medication in kidney transplant recipients: study protocol for a randomized controlled trial
Abstract
Background: Immunosuppression non-adherence in kidney transplant recipients (KTRs) not only increases the risk of medical intervention due to acute rejection and graft loss but burdens the socioeconomic system in the form of increased healthcare costs. An aggressive preemptive effort by healthcare professionals, geared to ensure adherence to immunosuppressants in KTRs, is significant and imperative.
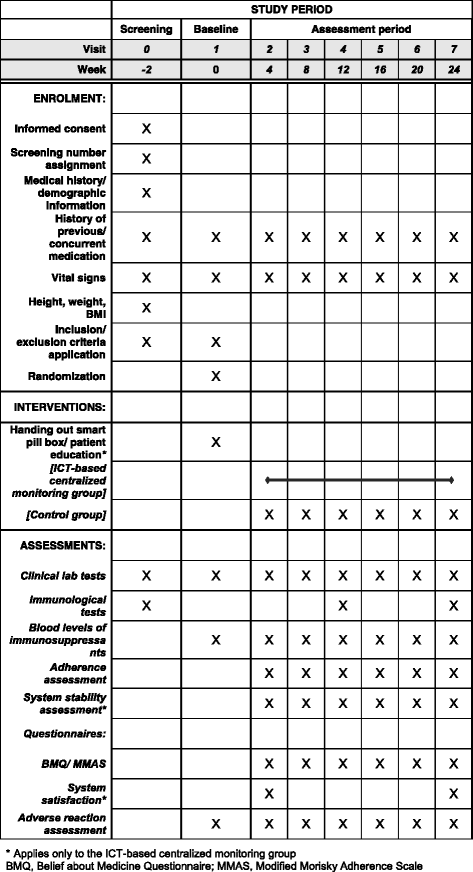
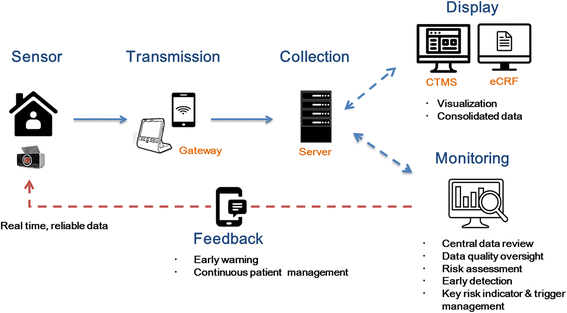
Methods/design: This study was designed as a prospective, open-label, multicenter, randomized controlled study aimed at evaluating the efficacy and stability of an information and communication technology (ICT)-based centralized monitoring system in boosting medication adherence in KTRs. One hundred fourteen KTRs registered throughout the year 2017 to 2018 are randomized into either the ICT-based centralized home monitoring system or to ambulatory follow-up. The planned follow-up duration is 6 months. The ICT-based centralized home monitoring system described consists of a smart pill box equipped with personal identification system, a home monitoring system, an electronic Case Report Form (eCRF) system, and a comprehensive clinical trial management system (CTMS). It alerts both patients and medical staff with texts and pill box alarms if there is a dosage/dosing time error or a missed dose. Medication adherence and transplant outcomes for the follow-up period are compared between the two groups, while patient satisfaction as well as the stability and cost-effectiveness of the ICT-based monitoring system are to be evaluated.
Discussion: This on-going study is expected to determine if consistent use of the ICT-based centralized monitoring system described could maximize mediation adherence and subsequently enhance transplant outcomes in KTRs. Further, it would lay the foundation for successful implementation of this ICT-based monitoring system for effective management of medication adherence in KTRs.
Trials registration: ClinicalTrials.gov, Identifier: NCT03136588 . Registered on 20 April 2017.
Keywords: Adherence; Information and communication technology; Kidney transplantation.
Conflict of interest statement
Ethics approval and consent to participate
All patients provided their written informed consent before participating in the study and the Daegu Joint Institutional Review Board approved the study protocol (DGIRB 2016-08-010). All clinical investigations were conducted in accordance with the guidelines of the Declaration of Helsinki and the Good Clinical Practice guidelines.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

