A systematic investigation of the maximum tolerated dose of cytotoxic chemotherapy with and without supportive care in mice
- PMID: 29037232
- PMCID: PMC5644108
- DOI: 10.1186/s12885-017-3677-7
A systematic investigation of the maximum tolerated dose of cytotoxic chemotherapy with and without supportive care in mice
Abstract
Background: Cytotoxic chemotherapeutics form the cornerstone of systemic treatment of many cancers. Patients are dosed at maximum tolerated dose (MTD), which is carefully determined in phase I studies. In contrast, in murine studies, dosages are often based on customary practice or small pilot studies, which often are not well documented. Consequently, research groups need to replicate experiments, resulting in an excess use of animals and highly variable dosages across the literature. In addition, while patients often receive supportive treatments in order to allow dose escalation, mice do not. These issues could affect experimental results and hence clinical translation.
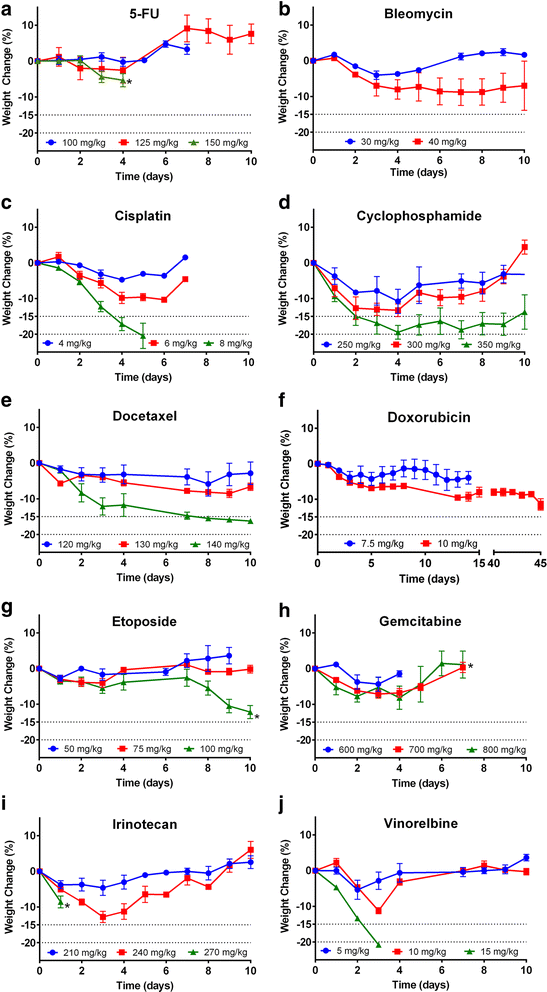
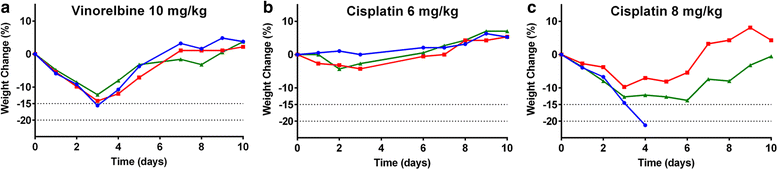
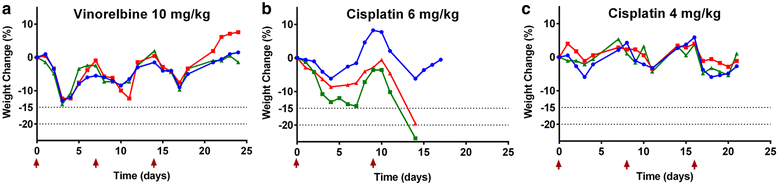
Methods: To address this, we determined the single-dose MTD in BALB/c and C57BL/6 mice for a range of chemotherapeutics covering the canonical classes, with clinical score and weight as endpoints.
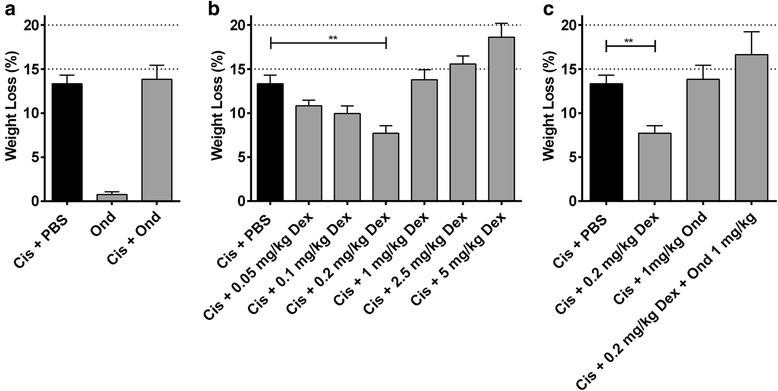
Results: We found that there was some variation in MTDs between strains and the tolerability of repeated cycles of chemotherapy at MTD was drug-dependent. We also demonstrate that dexamethasone reduces chemotherapy-induced weight loss in mice.
Conclusion: These data form a resource for future studies using chemotherapy in mice, increasing comparability between studies, reducing the number of mice needed for dose optimisation experiments and potentially improving translation to the clinic.
Keywords: Cancer; Chemotherapy; Dose optimization; MTD; Maximum tolerated dose; Mice; Supportive care.
Conflict of interest statement
Ethics approval
All experiments were conducted according to the University of Western Australia Animal Ethics Committee approvals (Protocols RA/3/100/1139, RA/3/100/1217) and the Harry Perkins Institute for Medical Research Animal Ethics Committee (AEC029–2015) and the code of conduct of the National Health and Medical Research Council of Australia.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Aston WJ, Fisher SA, Khong A, Mok C, Nowak AK, Lake RA, Lesterhuis WJ. Combining chemotherapy and checkpoint blockade in thoracic cancer: how to proceed? Lung Cancer Manag. 2014;3(6):443–457. doi: 10.2217/lmt.14.37. - DOI
-
- Hryniuk WM, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2(11)1281–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

