Caenorhabditis elegans Dosage Compensation: Insights into Condensin-Mediated Gene Regulation
- PMID: 29037439
- PMCID: PMC5741500
- DOI: 10.1016/j.tig.2017.09.010
Caenorhabditis elegans Dosage Compensation: Insights into Condensin-Mediated Gene Regulation
Abstract
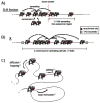
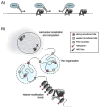
Recent work demonstrating the role of chromosome organization in transcriptional regulation has sparked substantial interest in the molecular mechanisms that control chromosome structure. Condensin, an evolutionarily conserved multisubunit protein complex, is essential for chromosome condensation during cell division and functions in regulating gene expression during interphase. In Caenorhabditis elegans, a specialized condensin forms the core of the dosage compensation complex (DCC), which specifically binds to and represses transcription from the hermaphrodite X chromosomes. DCC serves as a clear paradigm for addressing how condensins target large chromosomal domains and how they function to regulate chromosome structure and transcription. Here, we discuss recent research on C. elegans DCC in the context of canonical condensin mechanisms as have been studied in various organisms.
Keywords: X chromosome; condensin; cooperation; dosage compensation; gene regulation; genome organization.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


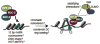
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

