PARP inhibitors: Clinical utility and possibilities of overcoming resistance
- PMID: 29037806
- PMCID: PMC5698126
- DOI: 10.1016/j.ygyno.2017.10.003
PARP inhibitors: Clinical utility and possibilities of overcoming resistance
Abstract
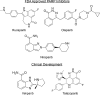
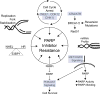
PARP inhibitors represent a major breakthrough in ovarian cancer care. Almost half of all ovarian cancers have deficiencies in the homologous recombination (HR) DNA repair pathway, namely BRCA1/2 mutations. Given the limited therapeutic options for recurrent ovarian cancer patients there has been a significant effort to develop novel therapies to exploit DNA repair deficiencies. In 2005 and 2006, inhibiting PARP enzymes was first observed to be highly effective against cancers with HR deficiencies. PARP inhibitors are being utilized in the clinic to manage recurrent ovarian cancers that display defects in the HR repair pathway. However, PARP inhibitors also show significant clinical benefit in patients without HR deficiencies. There are currently three FDA-approved PARP inhibitors for recurrent ovarian cancer and an additional two PARP inhibitors being evaluated in late stage clinical trials. Given the expanding clinical use of PARP inhibitors and the high likelihood of acquired resistance, there is a significant need for clinical strategies to manage PARP inhibitor resistant disease. This review will examine PARP inhibitors in the context of: indications and toxicities, novel biomarkers to predict response, targeted-therapy resistance, and potential approaches to manage resistant disease.
Keywords: BRCA1/2 mutation; Ovarian cancer; PARP inhibitors; Therapy resistance.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

