Will Systems Biology Deliver Its Promise and Contribute to the Development of New or Improved Vaccines? From Data to Understanding through Systems Biology
- PMID: 29038113
- PMCID: PMC5902663
- DOI: 10.1101/cshperspect.a028894
Will Systems Biology Deliver Its Promise and Contribute to the Development of New or Improved Vaccines? From Data to Understanding through Systems Biology
Abstract
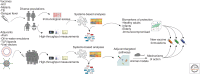
The advent of high-throughput "omics" technologies, combined with the computational and statistical methods necessary to analyze such data, have revolutionized biology, enabling a global view of the complex molecular processes and interactions that occur within a biological system. Such systems-based approaches have begun to be used in the evaluation of immune responses to vaccination, with the promise of identifying predictive biomarkers capable of rapidly evaluating vaccine efficacy, transforming our understanding of the immune mechanisms responsible for protective responses to vaccination and contributing to a new generation of rationally designed vaccines. Here we present our opinion that systems biology does indeed have a critical role in the future of vaccinology. Such approaches have shown potential in identifying transcriptional and cellular signatures of responsiveness to vaccination using diverse vaccines, adjuvants, and human populations. These findings, coupled with further mechanistic evaluation in animal models, will guide development of targeted vaccine and adjuvant formulations designed to optimally induce protective responses in populations of differing immune status.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
