Lactobacillus rhamnosus L34 Attenuates Gut Translocation-Induced Bacterial Sepsis in Murine Models of Leaky Gut
- PMID: 29038123
- PMCID: PMC5736799
- DOI: 10.1128/IAI.00700-17
Lactobacillus rhamnosus L34 Attenuates Gut Translocation-Induced Bacterial Sepsis in Murine Models of Leaky Gut
Abstract
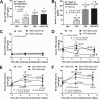
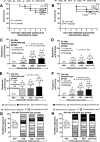
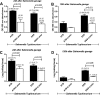
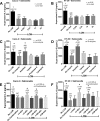
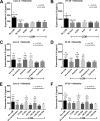
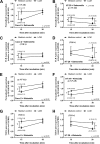
Gastrointestinal (GI) bacterial translocation in sepsis is well known, but the role of Lactobacillus species probiotics is still controversial. We evaluated the therapeutic effects of Lactobacillus rhamnosus L34 in a new sepsis model of oral administration of pathogenic bacteria with GI leakage induced by either an antibiotic cocktail (ATB) and/or dextran sulfate sodium (DSS). GI leakage with ATB, DSS, and DSS plus ATB (DSS+ATB) was demonstrated by fluorescein isothiocyanate (FITC)-dextran translocation to the circulation. The administration of pathogenic bacteria, either Klebsiella pneumoniae or Salmonella enterica serovar Typhimurium, enhanced translocation. Bacteremia was demonstrated within 24 h in 50 to 88% of mice with GI leakage plus the administration of pathogenic bacteria but not with GI leakage induction alone or bacterial gavage alone. Salmonella bacteremia was found in only 16 to 29% and 0% of mice with Salmonella and Klebsiella administrations, respectively. Klebsiella bacteremia was demonstrated in 25 to 33% and 10 to 16% of mice with Klebsiella and Salmonella administrations, respectively. Lactobacillus rhamnosus L34 attenuated GI leakage in these models, as shown by the reductions of FITC-dextran gut translocation, serum interleukin-6 (IL-6) levels, bacteremia, and sepsis mortality. The reduction in the amount of fecal Salmonella bacteria with Lactobacillus treatment was demonstrated. In addition, an anti-inflammatory effect of the conditioned medium from Lactobacillus rhamnosus L34 was also demonstrated by the attenuation of cytokine production in colonic epithelial cells in vitro In conclusion, Lactobacillus rhamnosus L34 attenuated the severity of symptoms in a murine sepsis model induced by GI leakage and the administration of pathogenic bacteria.
Keywords: Lactobacillus rhamnosus L34; antibiotics; dextran sulfate solution; gastrointestinal leakage; murine model.
Copyright © 2017 American Society for Microbiology.
Figures




Similar articles
-
Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34.Gut Microbes. 2020 May 3;11(3):465-480. doi: 10.1080/19490976.2019.1662712. Epub 2019 Sep 18. Gut Microbes. 2020. PMID: 31530137 Free PMC article.
-
Lactobacillus rhamnosus attenuates Thai chili extracts induced gut inflammation and dysbiosis despite capsaicin bactericidal effect against the probiotics, a possible toxicity of high dose capsaicin.PLoS One. 2021 Dec 23;16(12):e0261189. doi: 10.1371/journal.pone.0261189. eCollection 2021. PLoS One. 2021. PMID: 34941893 Free PMC article.
-
Lactobacillus rhamnosus L34 attenuates chronic kidney disease progression in a 5/6 nephrectomy mouse model through the excretion of anti-inflammatory molecules.Nephrol Dial Transplant. 2022 Jul 26;37(8):1429-1442. doi: 10.1093/ndt/gfac032. Nephrol Dial Transplant. 2022. PMID: 35138387
-
Breakthrough Lactobacillus rhamnosus GG bacteremia associated with probiotic use in an adult patient with severe active ulcerative colitis: case report and review of the literature.Infection. 2015 Dec;43(6):777-81. doi: 10.1007/s15010-015-0798-2. Epub 2015 May 30. Infection. 2015. PMID: 26024568 Review.
-
Lactobacillus Bacteremia and Probiotics: A Review.Microorganisms. 2023 Mar 30;11(4):896. doi: 10.3390/microorganisms11040896. Microorganisms. 2023. PMID: 37110319 Free PMC article. Review.
Cited by
-
Kazachstania pintolopesii in Blood and Intestinal Wall of Macrophage-Depleted Mice with Cecal Ligation and Puncture, the Control of Fungi by Macrophages during Sepsis.J Fungi (Basel). 2023 Dec 4;9(12):1164. doi: 10.3390/jof9121164. J Fungi (Basel). 2023. PMID: 38132765 Free PMC article.
-
Evidences and perspectives on the association between gut microbiota and sepsis: A bibliometric analysis from 2003 to 2023.Heliyon. 2024 Sep 14;10(18):e37921. doi: 10.1016/j.heliyon.2024.e37921. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39315201 Free PMC article.
-
Potential Utility of Induced Translocation of Engineered Bacteria as a Therapeutic Agent for Mounting a Personalized Neoantigen-Based Tumor Immune Response.Glob Chall. 2021 Dec 16;6(3):2100051. doi: 10.1002/gch2.202100051. eCollection 2022 Mar. Glob Chall. 2021. PMID: 35284089 Free PMC article.
-
Lactiplantibacillus plantarum dfa1 Outperforms Enterococcus faecium dfa1 on Anti-Obesity in High Fat-Induced Obesity Mice Possibly through the Differences in Gut Dysbiosis Attenuation, despite the Similar Anti-Inflammatory Properties.Nutrients. 2021 Dec 25;14(1):80. doi: 10.3390/nu14010080. Nutrients. 2021. PMID: 35010955 Free PMC article.
-
Less Severe Polymicrobial Sepsis in Conditional mgmt-Deleted Mice Using LysM-Cre System, Impacts of DNA Methylation and MGMT Inhibitor in Sepsis.Int J Mol Sci. 2023 Jun 15;24(12):10175. doi: 10.3390/ijms241210175. Int J Mol Sci. 2023. PMID: 37373325 Free PMC article.
References
-
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. doi:10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Leelahavanichkul A, Panpetch W, Worasilchai N, Somparn P, Chancharoenthana W, Nilgate S, Finkelman M, Chindamporn A, Tumwasorn S. 2016. Evaluation of gastrointestinal leakage using serum (1→3)-beta-d-glucan in a Clostridium difficile murine model. FEMS Microbiol Lett 363:fnw204. doi:10.1093/femsle/fnw204. - DOI - PubMed
-
- Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, Jutivorakool K, Somparn P, Issara-Amphorn J, Tachaboon S, Srisawat N, Finkelman M, Chindamporn A. 2016. Gastrointestinal leakage detected by serum (1→3)-beta-d-glucan in mouse models and a pilot study in patients with sepsis. Shock 46:506–518. doi:10.1097/SHK.0000000000000645. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

