Microbial Composition Predicts Genital Tract Inflammation and Persistent Bacterial Vaginosis in South African Adolescent Females
- PMID: 29038128
- PMCID: PMC5736802
- DOI: 10.1128/IAI.00410-17
Microbial Composition Predicts Genital Tract Inflammation and Persistent Bacterial Vaginosis in South African Adolescent Females
Abstract
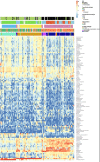
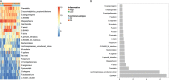
Young African females are at an increased risk of HIV acquisition, and genital inflammation or the vaginal microbiome may contribute to this risk. We studied these factors in 168 HIV-negative South African adolescent females aged 16 to 22 years. Unsupervised clustering of 16S rRNA gene sequences revealed three clusters (subtypes), one of which was strongly associated with genital inflammation. In a multivariate model, the microbiome compositional subtype and hormonal contraception were significantly associated with genital inflammation. We identified 40 taxa significantly associated with inflammation, including those reported previously (Prevotella, Sneathia, Aerococcus, Fusobacterium, and Gemella) as well as several novel taxa (including increased frequencies of bacterial vaginosis-associated bacterium 1 [BVAB1], BVAB2, BVAB3, Prevotella amnii, Prevotella pallens, Parvimonas micra, Megasphaera, Gardnerella vaginalis, and Atopobium vaginae and decreased frequencies of Lactobacillus reuteri, Lactobacillus crispatus, Lactobacillus jensenii, and Lactobacillus iners). Women with inflammation-associated microbiomes had significantly higher body mass indices and lower levels of endogenous estradiol and luteinizing hormone. Community functional profiling revealed three distinct vaginal microbiome subtypes, one of which was characterized by extreme genital inflammation and persistent bacterial vaginosis (BV); this subtype could be predicted with high specificity and sensitivity based on the Nugent score (≥9) or BVAB1 abundance. We propose that women with this BVAB1-dominated subtype may have chronic genital inflammation due to persistent BV, which may place them at a particularly high risk for HIV infection.
Keywords: 16S RNA; HIV susceptibility; HIV target cells; female genital tract microbiome; inflammation; vaginal microbiome.
Copyright © 2017 American Society for Microbiology.
Figures





References
-
- Masson L, Passmore JS, Liebenberg LJ, Werner L, Baxter C, Arnold KB, Williamson C, Little F, Mansoor LE, Naranbhai V, Lauffenburger DA, Ronacher K, Walzl G, Garrett NJ, Williams BL, Couto-Rodriguez M, Hornig M, Lipkin WI, Grobler A, Abdool Karim Q, Abdool Karim SS. 2015. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 61:260–269. doi:10.1093/cid/civ298. - DOI - PMC - PubMed
-
- Deese J, Masson L, Miller W, Cohen M, Morrison C, Wang M, Ahmed K, Agot K, Crucitti T, Abdellati S, Van Damme L. 2015. Injectable progestin-only contraception is associated with increased levels of pro-inflammatory cytokines in the female genital tract. Am J Reprod Immunol 74:357–367. doi:10.1111/aji.12415. - DOI - PubMed
-
- Morrison CS, Chen P-L, Kwok C, Baeten JM, Brown J, Crook AM, Van Damme L, Delany-Moretlwe S, Francis SC, Friedland BA, Hayes RJ, Heffron R, Kapiga S, Karim QA, Karpoff S, Kaul R, McClelland RS, McCormack S, McGrath N, Myer L, Rees H, van der Straten A, Watson-Jones D, van de Wijgert JHHM, Stalter R, Low N. 2015. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med 12:e1001778. doi:10.1371/journal.pmed.1001778. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

