Loss of Extracellular Signal-Regulated Kinase 1/2 in the Retinal Pigment Epithelium Leads to RPE65 Decrease and Retinal Degeneration
- PMID: 29038159
- PMCID: PMC5705814
- DOI: 10.1128/MCB.00295-17
Loss of Extracellular Signal-Regulated Kinase 1/2 in the Retinal Pigment Epithelium Leads to RPE65 Decrease and Retinal Degeneration
Abstract
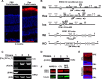
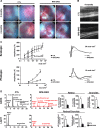
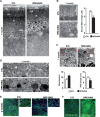
Recent work suggested that the activity of extracellular signal-regulated kinase 1/2 (ERK1/2) is increased in the retinal pigment epithelium (RPE) of age-related macular degeneration (ARMD) patients and therefore could be an attractive therapeutic target. Notably, ERK1/2 pathway inhibitors are used in cancer therapy, with severe and noncharacterized ocular side effects. To decipher the role of ERK1/2 in RPE cells, we conditionally disrupted the Erk1 and Erk2 genes in mouse RPE. The loss of ERK1/2 activity resulted in a significant decrease in the level of RPE65 expression, a decrease in ocular retinoid levels concomitant with low visual function, and a rapid disorganization of RPE cells, ultimately leading to retinal degeneration. Our results identify the ERK1/2 pathway as a direct regulator of the visual cycle and a critical component of the viability of RPE and photoreceptor cells. Moreover, our results caution about the need for a very fine adjustment of kinase inhibition in cancer or ARMD treatment in order to avoid ocular side effects.
Keywords: AP-1; ERK1/2; RPE65; electron microscopy; photoreceptors; retinal degeneration; retinoid.
Copyright © 2017 Pyakurel et al.
Figures




Similar articles
-
The novel visual cycle inhibitor (±)-RPE65-61 protects retinal photoreceptors from light-induced degeneration.PLoS One. 2022 Oct 13;17(10):e0269437. doi: 10.1371/journal.pone.0269437. eCollection 2022. PLoS One. 2022. PMID: 36227868 Free PMC article.
-
RPE Visual Cycle and Biochemical Phenotypes of Mutant Mouse Models.Methods Mol Biol. 2018;1753:89-102. doi: 10.1007/978-1-4939-7720-8_6. Methods Mol Biol. 2018. PMID: 29564783
-
Inhibition of the visual cycle by A2E through direct interaction with RPE65 and implications in Stargardt disease.Proc Natl Acad Sci U S A. 2010 Oct 12;107(41):17551-6. doi: 10.1073/pnas.1008769107. Epub 2010 Sep 27. Proc Natl Acad Sci U S A. 2010. PMID: 20876139 Free PMC article.
-
Retinoid Processing in Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cultures.Prog Mol Biol Transl Sci. 2015;134:477-90. doi: 10.1016/bs.pmbts.2015.06.004. Epub 2015 Jul 9. Prog Mol Biol Transl Sci. 2015. PMID: 26310172 Free PMC article. Review.
-
Retinal pigment epithelium 65 kDa protein (RPE65): An update.Prog Retin Eye Res. 2022 May;88:101013. doi: 10.1016/j.preteyeres.2021.101013. Epub 2021 Oct 2. Prog Retin Eye Res. 2022. PMID: 34607013 Free PMC article. Review.
Cited by
-
Glabridin Attenuates the Retinal Degeneration Induced by Sodium Iodate In Vitro and In Vivo.Front Pharmacol. 2020 Oct 15;11:566699. doi: 10.3389/fphar.2020.566699. eCollection 2020. Front Pharmacol. 2020. PMID: 33178017 Free PMC article.
-
Quantitative multi-contrast in vivo mouse imaging with polarization diversity optical coherence tomography and angiography.Biomed Opt Express. 2020 Nov 6;11(12):6945-6961. doi: 10.1364/BOE.403209. eCollection 2020 Dec 1. Biomed Opt Express. 2020. PMID: 33408972 Free PMC article.
-
Regulation of RPE65 expression in human retinal pigment epithelium cells.Sci Rep. 2025 Jul 25;15(1):27106. doi: 10.1038/s41598-025-12926-3. Sci Rep. 2025. PMID: 40715346 Free PMC article.
-
The cGMP system in normal and degenerating mouse neuroretina: New proteins with cGMP interaction potential identified by a proteomics approach.J Neurochem. 2021 Jun;157(6):2173-2186. doi: 10.1111/jnc.15251. Epub 2020 Dec 5. J Neurochem. 2021. PMID: 33230839 Free PMC article.
-
Porcine Single-Eye Retinal Pigment Epithelium Cell Culture for Barrier and Polarity Studies.Cells. 2025 Jul 1;14(13):1007. doi: 10.3390/cells14131007. Cells. 2025. PMID: 40643526 Free PMC article.
References
-
- Hageman GS, Luthert PJ, Chong NHV, Johnson LV, Anderson DH, Mullins RF. 2001. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 20:705–732. doi:10.1016/S1350-9462(01)00010-6. - DOI - PubMed
-
- Iriyama A, Fujiki R, Inoue Y, Takahashi H, Tamaki Y, Takezawa S, Takeyama K, Jang WD, Kato S, Yanagi Y. 2008. A2E, a pigment of the lipofuscin of retinal pigment epithelial cells, is an endogenous ligand for retinoic acid receptor. J Biol Chem 283:11947–11953. doi:10.1074/jbc.M708989200. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
