Protein Kinase A/CREB Signaling Prevents Adriamycin-Induced Podocyte Apoptosis via Upregulation of Mitochondrial Respiratory Chain Complexes
- PMID: 29038164
- PMCID: PMC5730724
- DOI: 10.1128/MCB.00181-17
Protein Kinase A/CREB Signaling Prevents Adriamycin-Induced Podocyte Apoptosis via Upregulation of Mitochondrial Respiratory Chain Complexes
Abstract
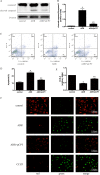
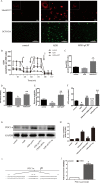
Previous work showed that the activation of protein kinase A (PKA) signaling promoted mitochondrial fusion and prevented podocyte apoptosis. The cAMP response element binding protein (CREB) is the main downstream transcription factor of PKA signaling. Here we show that the PKA agonist 8-(4-chlorophenylthio)adenosine 3',5'-cyclic monophosphate-cyclic AMP (pCPT-cAMP) prevented the production of adriamycin (ADR)-induced reactive oxygen species and apoptosis in podocytes, which were inhibited by CREB RNA interference (RNAi). The activation of PKA enhanced mitochondrial function and prevented the ADR-induced decrease of mitochondrial respiratory chain complex I subunits, NADH-ubiquinone oxidoreductase complex (ND) 1/3/4 genes, and protein expression. Inhibition of CREB expression alleviated pCPT-cAMP-induced ND3, but not the recovery of ND1/4 protein, in ADR-treated podocytes. In addition, CREB RNAi blocked the pCPT-cAMP-induced increase in ATP and the expression of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1-α). The chromatin immunoprecipitation assay showed enrichment of CREB on PGC1-α and ND3 promoters, suggesting that these promoters are CREB targets. In vivo, both an endogenous cAMP activator (isoproterenol) and pCPT-cAMP decreased the albumin/creatinine ratio in mice with ADR nephropathy, reduced glomerular oxidative stress, and retained Wilm's tumor suppressor gene 1 (WT-1)-positive cells in glomeruli. We conclude that the upregulation of mitochondrial respiratory chain proteins played a partial role in the protection of PKA/CREB signaling.
Keywords: CREB; PKA signaling; apoptosis; mitochondria; podocyte.
Copyright © 2017 American Society for Microbiology.
Figures




Similar articles
-
cAMP signaling prevents podocyte apoptosis via activation of protein kinase A and mitochondrial fusion.PLoS One. 2014 Mar 18;9(3):e92003. doi: 10.1371/journal.pone.0092003. eCollection 2014. PLoS One. 2014. PMID: 24642777 Free PMC article.
-
Overexpression of human selenoprotein H in neuronal cells enhances mitochondrial biogenesis and function through activation of protein kinase A, protein kinase B, and cyclic adenosine monophosphate response element-binding protein pathway.Int J Biochem Cell Biol. 2013 Mar;45(3):604-11. doi: 10.1016/j.biocel.2012.11.022. Epub 2012 Dec 7. Int J Biochem Cell Biol. 2013. PMID: 23220172 Free PMC article.
-
Cyclic AMP-mediated upregulation of the expression of neuronal NO synthase in human A673 neuroepithelioma cells results in a decrease in the level of bioactive NO production: analysis of the signaling mechanisms that are involved.Biochemistry. 2004 Jun 8;43(22):7197-206. doi: 10.1021/bi0302191. Biochemistry. 2004. PMID: 15170357
-
Respiratory chain complex I, a main regulatory target of the cAMP/PKA pathway is defective in different human diseases.FEBS Lett. 2012 Mar 9;586(5):568-77. doi: 10.1016/j.febslet.2011.09.019. Epub 2011 Sep 19. FEBS Lett. 2012. PMID: 21945319 Review.
-
Mitochondrial cAMP-PKA signaling: What do we really know?Biochim Biophys Acta Bioenerg. 2018 Sep;1859(9):868-877. doi: 10.1016/j.bbabio.2018.04.005. Epub 2018 Apr 23. Biochim Biophys Acta Bioenerg. 2018. PMID: 29694829 Review.
Cited by
-
Contrasting consequences of podocyte insulin-like growth factor 1 receptor inhibition.iScience. 2024 Apr 16;27(5):109749. doi: 10.1016/j.isci.2024.109749. eCollection 2024 May 17. iScience. 2024. PMID: 38706850 Free PMC article.
-
Early life exposures shape the CD4+ T cell transcriptome, influencing proliferation, differentiation, and mitochondrial dynamics later in life.Sci Rep. 2019 Aug 7;9(1):11489. doi: 10.1038/s41598-019-47866-2. Sci Rep. 2019. PMID: 31391494 Free PMC article.
-
β2-Adrenergic receptor agonism as a therapeutic strategy for kidney disease.Am J Physiol Regul Integr Comp Physiol. 2021 May 1;320(5):R575-R587. doi: 10.1152/ajpregu.00287.2020. Epub 2021 Feb 10. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 33565369 Free PMC article. Review.
-
The complicated role of mitochondria in the podocyte.Am J Physiol Renal Physiol. 2020 Dec 1;319(6):F955-F965. doi: 10.1152/ajprenal.00393.2020. Epub 2020 Oct 19. Am J Physiol Renal Physiol. 2020. PMID: 33073585 Free PMC article.
-
Therapeutic Potential Targeting Podocyte Mitochondrial Dysfunction in Focal Segmental Glomerulosclerosis.Kidney Dis (Basel). 2023 Mar 28;9(4):254-264. doi: 10.1159/000530344. eCollection 2023 Aug. Kidney Dis (Basel). 2023. PMID: 37900001 Free PMC article. Review.
References
-
- Maezawa Y, Onay T, Scott RP, Keir LS, Dimke H, Li C, Eremina V, Maezawa Y, Jeansson M, Shan J, Binnie M, Lewin M, Ghosh A, Miner JH, Vainio SJ, Quaggin SE. 2014. Loss of the podocyte-expressed transcription factor Tcf21/Pod1 results in podocyte differentiation defects and FSGS. J Am Soc Nephrol 25:2459–2470. doi: 10.1681/ASN.2013121307. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
