Context memory formation requires activity-dependent protein degradation in the hippocampus
- PMID: 29038220
- PMCID: PMC5647928
- DOI: 10.1101/lm.045443.117
Context memory formation requires activity-dependent protein degradation in the hippocampus
Abstract
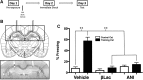
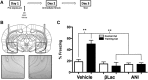
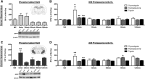
Numerous studies have indicated that the consolidation of contextual fear memories supported by an aversive outcome like footshock requires de novo protein synthesis as well as protein degradation mediated by the ubiquitin-proteasome system (UPS). Context memory formed in the absence of an aversive stimulus by simple exposure to a novel environment requires de novo protein synthesis in both the dorsal (dHPC) and ventral (vHPC) hippocampus. However, the role of UPS-mediated protein degradation in the consolidation of context memory in the absence of a strong aversive stimulus has not been investigated. In the present study, we used the context preexposure facilitation effect (CPFE) procedure, which allows for the dissociation of context learning from context-shock learning, to investigate the role of activity-dependent protein degradation in the dHPC and vHPC during the formation of a context memory. We report that blocking protein degradation with the proteasome inhibitor clasto-lactacystin β-lactone (βLac) or blocking protein synthesis with anisomycin (ANI) immediately after context preexposure significantly impaired context memory formation. Additionally, we examined 20S proteasome activity at different time points following context exposure and saw that the activity of proteasomes in the dHPC increases immediately after stimulus exposure while the vHPC exhibits a biphasic pattern of proteolytic activity. Taken together, these data suggest that the requirement of increased proteolysis during memory consolidation is not driven by processes triggered by the strong aversive outcome (i.e., shock) normally used to support fear conditioning.
© 2017 Cullen et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

Similar articles
-
Medial prefrontal and ventral hippocampal contributions to incidental context learning and memory in adolescent rats.Neurobiol Learn Mem. 2019 Dec;166:107091. doi: 10.1016/j.nlm.2019.107091. Epub 2019 Sep 19. Neurobiol Learn Mem. 2019. PMID: 31542328
-
Antagonism of muscarinic acetylcholine receptors in medial prefrontal cortex disrupts the context preexposure facilitation effect.Neurobiol Learn Mem. 2017 Sep;143:27-35. doi: 10.1016/j.nlm.2017.04.003. Epub 2017 Apr 11. Neurobiol Learn Mem. 2017. PMID: 28411153 Free PMC article.
-
The relationship between protein synthesis and protein degradation in object recognition memory.Behav Brain Res. 2015 Nov 1;294:17-24. doi: 10.1016/j.bbr.2015.07.038. Epub 2015 Jul 19. Behav Brain Res. 2015. PMID: 26200717
-
The Ubiquitin-Proteasome System and Memory: Moving Beyond Protein Degradation.Neuroscientist. 2018 Dec;24(6):639-651. doi: 10.1177/1073858418762317. Epub 2018 Mar 12. Neuroscientist. 2018. PMID: 29529924 Review.
-
Contextual fear, gestalt memories, and the hippocampus.Behav Brain Res. 2000 Jun 1;110(1-2):73-81. doi: 10.1016/s0166-4328(99)00186-2. Behav Brain Res. 2000. PMID: 10802305 Review.
Cited by
-
Sex differences in training-induced activity of the ubiquitin proteasome system in the dorsal hippocampus and medial prefrontal cortex of male and female mice.Learn Mem. 2022 Sep 2;29(9):302-311. doi: 10.1101/lm.053492.121. Print 2022 Sep. Learn Mem. 2022. PMID: 36206392 Free PMC article.
-
Identification of Ndfip1 as a novel negative regulator for spatial memory formation associated with increased ubiquitination of Beclin 1 and PTEN.PLoS One. 2023 Apr 6;18(4):e0283908. doi: 10.1371/journal.pone.0283908. eCollection 2023. PLoS One. 2023. PMID: 37023120 Free PMC article.
-
Coordinated transcriptional regulation by thyroid hormone and glucocorticoid interaction in adult mouse hippocampus-derived neuronal cells.PLoS One. 2019 Jul 26;14(7):e0220378. doi: 10.1371/journal.pone.0220378. eCollection 2019. PLoS One. 2019. PMID: 31348800 Free PMC article.
-
Dysregulation of protein degradation in the hippocampus is associated with impaired spatial memory during the development of obesity.Behav Brain Res. 2020 Sep 1;393:112787. doi: 10.1016/j.bbr.2020.112787. Epub 2020 Jun 27. Behav Brain Res. 2020. PMID: 32603798 Free PMC article.
-
Unique roles for the anterior and posterior retrosplenial cortices in encoding and retrieval of memory for context.Cereb Cortex. 2022 Aug 22;32(17):3602-3610. doi: 10.1093/cercor/bhab436. Cereb Cortex. 2022. PMID: 35029643 Free PMC article.
References
-
- Artinian J, McGauran AMT, De Jaeger X, Mouledous L, Frances B, Roullet P. 2008. Protein degradation, as with protein synthesis, is required during not only long-term spatial memory consolidation but also reconsolidation. Eur J Neurosci 27: 3009–3019. - PubMed
-
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. 1999. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behav Neurosci 113: 276–282. - PubMed
-
- Banerjee S, Neveu P, Kosik KS. 2009. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron 64: 871–884. - PubMed
-
- Barrientos RM, O'Reilly RC, Rudy JW. 2002. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res 134: 299–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
