Constitutive activity of the Ghrelin receptor reduces surface expression of voltage-gated Ca2+ channels in a CaVβ-dependent manner
- PMID: 29038230
- PMCID: PMC6518300
- DOI: 10.1242/jcs.207886
Constitutive activity of the Ghrelin receptor reduces surface expression of voltage-gated Ca2+ channels in a CaVβ-dependent manner
Abstract
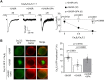
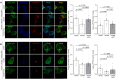
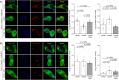
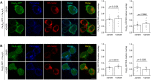
Voltage-gated Ca2+ (CaV) channels couple membrane depolarization to Ca2+ influx, triggering a range of Ca2+-dependent cellular processes. CaV channels are, therefore, crucial in shaping neuronal activity and function, depending on their individual temporal and spatial properties. Furthermore, many neurotransmitters and drugs that act through G protein coupled receptors (GPCRs), modulate neuronal activity by altering the expression, trafficking, or function of CaV channels. GPCR-dependent mechanisms that downregulate CaV channel expression levels are observed in many neurons but are, by comparison, less studied. Here we show that the growth hormone secretagogue receptor type 1a (GHSR), a GPCR, can inhibit the forwarding trafficking of several CaV subtypes, even in the absence of agonist. This constitutive form of GPCR inhibition of CaV channels depends on the presence of a CaVβ subunit. CaVβ subunits displace CaVα1 subunits from the endoplasmic reticulum. The actions of GHSR on CaV channels trafficking suggest a role for this signaling pathway in brain areas that control food intake, reward, and learning and memory.
Keywords: CaVβ; GPCR; Voltage-gated calcium (Ca2+) channels.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




Similar articles
-
Growth hormone secretagogue receptor constitutive activity impairs voltage-gated calcium channel-dependent inhibitory neurotransmission in hippocampal neurons.J Physiol. 2018 Nov;596(22):5415-5428. doi: 10.1113/JP276256. Epub 2018 Oct 14. J Physiol. 2018. PMID: 30199095 Free PMC article.
-
Ghrelin reduces voltage-gated calcium currents in GH₃ cells via cyclic GMP pathways.Endocrine. 2011 Oct;40(2):228-36. doi: 10.1007/s12020-011-9520-z. Epub 2011 Aug 27. Endocrine. 2011. PMID: 21874320
-
Cavβ3 Regulates Ca2+ Signaling and Insulin Expression in Pancreatic β-Cells in a Cell-Autonomous Manner.Diabetes. 2021 Nov;70(11):2532-2544. doi: 10.2337/db21-0078. Epub 2021 Aug 23. Diabetes. 2021. PMID: 34426509 Free PMC article.
-
Ca2+-dependent modulation of voltage-gated Ca2+ channels.Biochim Biophys Acta. 2012 Aug;1820(8):1243-52. doi: 10.1016/j.bbagen.2011.12.012. Epub 2011 Dec 24. Biochim Biophys Acta. 2012. PMID: 22223119 Free PMC article. Review.
-
KCa and Ca(2+) channels: the complex thought.Biochim Biophys Acta. 2014 Oct;1843(10):2322-33. doi: 10.1016/j.bbamcr.2014.02.019. Epub 2014 Mar 6. Biochim Biophys Acta. 2014. PMID: 24613282 Review.
Cited by
-
Ninjin'yoeito Targets Distinct Ca2+ Channels to Activate Ghrelin-Responsive vs. Unresponsive NPY Neurons in the Arcuate Nucleus.Front Nutr. 2020 Jul 17;7:104. doi: 10.3389/fnut.2020.00104. eCollection 2020. Front Nutr. 2020. PMID: 32766273 Free PMC article.
-
Heal the heart through gut (hormone) ghrelin: a potential player to combat heart failure.Heart Fail Rev. 2021 Mar;26(2):417-435. doi: 10.1007/s10741-020-10032-2. Epub 2020 Oct 6. Heart Fail Rev. 2021. PMID: 33025414 Review.
-
Growth hormone secretagogue receptor constitutive activity impairs voltage-gated calcium channel-dependent inhibitory neurotransmission in hippocampal neurons.J Physiol. 2018 Nov;596(22):5415-5428. doi: 10.1113/JP276256. Epub 2018 Oct 14. J Physiol. 2018. PMID: 30199095 Free PMC article.
-
Constitutive activity of dopamine receptor type 1 (D1R) increases CaV2.2 currents in PFC neurons.J Gen Physiol. 2020 May 4;152(5):e201912492. doi: 10.1085/jgp.201912492. J Gen Physiol. 2020. PMID: 32259196 Free PMC article.
-
ACE2 internalization induced by a SARS-CoV-2 recombinant protein is modulated by angiotensin II type 1 and bradykinin 2 receptors.Life Sci. 2022 Mar 15;293:120284. doi: 10.1016/j.lfs.2021.120284. Epub 2022 Jan 14. Life Sci. 2022. PMID: 35038454 Free PMC article.
References
-
- Andrews Z. B., Erion D., Beiler R., Liu Z.-W., Abizaid A., Zigman J., Elsworth J. D., Savitt J. M., Dimarchi R., Tschoep M. et al. (2009). Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J. Neurosci. 29, 14057-14065. 10.1523/JNEUROSCI.3890-09.2009 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

