Tissue Force Programs Cell Fate and Tumor Aggression
- PMID: 29038232
- PMCID: PMC5679454
- DOI: 10.1158/2159-8290.CD-16-0733
Tissue Force Programs Cell Fate and Tumor Aggression
Abstract
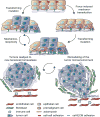
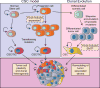
Biomechanical and biochemical cues within a tissue collaborate across length scales to direct cell fate during development and are critical for the maintenance of tissue homeostasis. Loss of tensional homeostasis in a tissue not only accompanies malignancy but may also contribute to oncogenic transformation. High mechanical stress in solid tumors can impede drug delivery and may additionally drive tumor progression and promote metastasis. Mechanistically, biomechanical forces can drive tumor aggression by inducing a mesenchymal-like switch in transformed cells so that they attain tumor-initiating or stem-like cell properties. Given that cancer stem cells have been linked to metastasis and treatment resistance, this raises the intriguing possibility that the elevated tissue mechanics in tumors could promote their aggression by programming their phenotype toward that exhibited by a stem-like cell.Significance: Recent findings argue that mechanical stress and elevated mechanosignaling foster malignant transformation and metastasis. Prolonged corruption of tissue tension may drive tumor aggression by altering cell fate specification. Thus, strategies that could reduce tumor mechanics might comprise effective approaches to prevent the emergence of treatment-resilient metastatic cancers. Cancer Discov; 7(11); 1224-37. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures

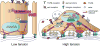
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

