Efficacy of Prophylactic Treatment for Oxycodone-Induced Nausea and Vomiting Among Patients with Cancer Pain (POINT): A Randomized, Placebo-Controlled, Double-Blind Trial
- PMID: 29038236
- PMCID: PMC5905679
- DOI: 10.1634/theoncologist.2017-0225
Efficacy of Prophylactic Treatment for Oxycodone-Induced Nausea and Vomiting Among Patients with Cancer Pain (POINT): A Randomized, Placebo-Controlled, Double-Blind Trial
Abstract
Background: Although opioid-induced nausea and vomiting (OINV) often result in analgesic undertreatment in patients with cancer, no randomized controlled trials have evaluated the efficacy of prophylactic antiemetics for preventing OINV. We conducted this randomized, placebo-controlled, double-blind trial to evaluate the efficacy and safety of prophylactic treatment with prochlorperazine for preventing OINV.
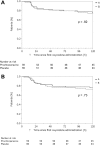
Materials and methods: Cancer patients who started to receive oral oxycodone were randomly assigned in a 1:1 ratio to receive either prochlorperazine 5 mg or placebo prophylactically, given three times daily for 5 days. The primary endpoint was the proportion of patients who had a complete response (CR) during the 120 hours of oxycodone treatment. CR was defined as no emetic episode and no use of rescue medication for nausea and vomiting during 5 days. Key secondary endpoints were the proportion of patients with emetic episodes, proportion of patients with moderate or severe nausea, quality of life, and proportion of treatment withdrawal.
Results: From November 2013 through February 2016, a total of 120 patients were assigned to receive prochlorperazine (n = 60) or placebo (n = 60). There was no significant difference in CR rates (69.5% vs. 63.3%; p = .47) or any secondary endpoint between the groups. Patients who received prochlorperazine were more likely to experience severe somnolence (p = .048).
Conclusion: Routine use of prochlorperazine as a prophylactic antiemetic at the initiation of treatment with opioids is not recommended. Further research is needed to evaluate whether other antiemetics would be effective in preventing OINV in specific patient populations.
Implications for practice: Prophylactic prochlorperazine seems to be ineffective in preventing opioid-induced nausea and vomiting (OINV) and may cause adverse events such as somnolence. Routine use of prophylactic prochlorperazine at the initiation of treatment with opioids is not recommended. Further research is needed to evaluate whether other antiemetics would be effective in preventing OINV in specific patient populations.
Keywords: Antiemetics; Cancer pain; Opioid‐induced nausea and vomiting; Oxycodone; Prochlorperazine; Prophylaxis.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures


Similar articles
-
Prophylactic Use of Antiemetics for Prevention of Opioid-Induced Nausea and Vomiting: A Questionnaire Survey among Japanese Physicians.J Palliat Med. 2015 Nov;18(11):977-80. doi: 10.1089/jpm.2015.0203. Epub 2015 Aug 20. J Palliat Med. 2015. PMID: 26509390
-
Incidence of nausea and vomiting induced by oxycodone administered with prochlorperazine in Japanese cancer patients.J Nippon Med Sch. 2015;82(2):100-5. doi: 10.1272/jnms.82.100. J Nippon Med Sch. 2015. PMID: 25959201
-
Investigating the efficacy and safety of olanzapine prophylaxis for opioid-induced nausea and vomiting (JORTC-PAL20): a study protocol for an open-label, single-arm exploratory study.BMJ Open. 2024 Feb 27;14(2):e076575. doi: 10.1136/bmjopen-2023-076575. BMJ Open. 2024. PMID: 38417963 Free PMC article.
-
Economic and clinical burden of opioid-induced nausea and vomiting.Postgrad Med. 2017 Jan;129(1):111-117. doi: 10.1080/00325481.2017.1243004. Epub 2016 Oct 11. Postgrad Med. 2017. PMID: 27690715 Review.
-
Pharmacological antiemetic prophylaxis and treatment for opioid-induced nausea and vomiting (OINV) in patients treated for cancer pain and cancer-related breathlessness.Cochrane Database Syst Rev. 2025 Aug 29;8(8):CD016207. doi: 10.1002/14651858.CD016207. Cochrane Database Syst Rev. 2025. PMID: 40878845 Free PMC article.
Cited by
-
Management of pain in cancer patients - an update.Ecancermedicalscience. 2024 Dec 12;18:1821. doi: 10.3332/ecancer.2024.1821. eCollection 2024. Ecancermedicalscience. 2024. PMID: 40171458 Free PMC article.
-
The efficacy of prophylactic prochlorperazine injections at the initiation of opioid injections in preventing opioid-induced nausea and vomiting among patients with end-stage cancer.Fujita Med J. 2023 Nov;9(4):270-274. doi: 10.20407/fmj.2022-034. Epub 2023 Aug 28. Fujita Med J. 2023. PMID: 38077960 Free PMC article.
-
Opioid-Related Side Effects and Management.Cancer Treat Res. 2021;182:97-105. doi: 10.1007/978-3-030-81526-4_7. Cancer Treat Res. 2021. PMID: 34542878
-
Effect of Prophylactic Anti-emetics on Opioid-induced Nausea and Vomiting: A Retrospective Observational Cohort Study.In Vivo. 2021 May-Jun;35(3):1737-1742. doi: 10.21873/invivo.12432. In Vivo. 2021. PMID: 33910857 Free PMC article.
-
Impact of Opioid Consumption in Patients With Functional Gastrointestinal Disorders.Front Pharmacol. 2020 Dec 21;11:596467. doi: 10.3389/fphar.2020.596467. eCollection 2020. Front Pharmacol. 2020. PMID: 33414719 Free PMC article.
References
-
- van den Beuken‐van Everdingen MH, de Rijke JM, Kessels AG et al. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol 2007;18:1437–1449. - PubMed
-
- Breivik H, Cherny N, Collett B et al. Cancer‐related pain: A pan‐European survey of prevalence, treatment, and patient attitudes. Ann Oncol 2009;20:1420–1433. - PubMed
-
- van den Beuken‐van Everdingen MH, Hochstenbach LM, Joosten EA et al. Update on prevalence of pain in patients with cancer: Systematic review and meta‐analysis. J Pain Symptom Manage 2016;51:1070–1090. - PubMed
-
- Caraceni A, Hanks G, Kaasa S et al. Use of opioid analgesics in the treatment of cancer pain: Evidence‐based recommendation from the EAPC. Lancet Oncol 2012;13:e58–e68. - PubMed
-
- Ripamonti CI, Santini D, Maranzano E et al. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol 2012;23:139–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

