Correlated Disruption of Resting-State fMRI, LFP, and Spike Connectivity between Area 3b and S2 following Spinal Cord Injury in Monkeys
- PMID: 29038239
- PMCID: PMC5688527
- DOI: 10.1523/JNEUROSCI.2318-17.2017
Correlated Disruption of Resting-State fMRI, LFP, and Spike Connectivity between Area 3b and S2 following Spinal Cord Injury in Monkeys
Abstract
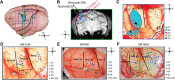
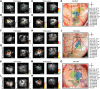
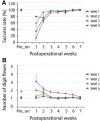
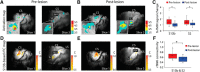
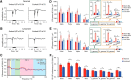
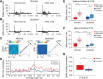
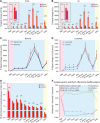
This study aims to understand how functional connectivity (FC) between areas 3b and S2 alters following input deprivation and the neuronal basis of disrupted FC of resting-state fMRI signals. We combined submillimeter fMRI with microelectrode recordings to localize the deafferented digit regions in areas 3b and S2 by mapping tactile stimulus-evoked fMRI activations before and after cervical dorsal column lesion in each male monkey. An average afferent disruption of 97% significantly reduced fMRI, local field potential (LFP), and spike responses to stimuli in both areas. Analysis of resting-state fMRI signal correlation, LFP coherence, and spike cross-correlation revealed significantly reduced functional connectivity between deafferented areas 3b and S2. The degrees of reductions in stimulus responsiveness and FC after deafferentation differed across fMRI, LFP, and spiking signals. The reduction of FC was much weaker than that of stimulus-evoked responses. Whereas the largest stimulus-evoked signal drop (∼80%) was observed in LFP signals, the greatest FC reduction was detected in the spiking activity (∼30%). fMRI signals showed mild reductions in stimulus responsiveness (∼25%) and FC (∼20%). The overall deafferentation-induced changes were quite similar in areas 3b and S2 across signals. Here we demonstrated that FC strength between areas 3b and S2 was much weakened by dorsal column lesion, and stimulus response reduction and FC disruption in fMRI covary with those of LFP and spiking signals in deafferented areas 3b and S2. These findings have important implications for fMRI studies aiming to probe FC alterations in pathological conditions involving deafferentation in humans.SIGNIFICANCE STATEMENT By directly comparing fMRI, local field potential, and spike signals in both tactile stimulation and resting states before and after severe disruption of dorsal column afferent, we demonstrated that reduction in fMRI responses to stimuli is accompanied by weakened resting-state fMRI functional connectivity (FC) in input-deprived and reorganized digit regions in area 3b of the S1 and S2. Concurrent reductions in local field potential and spike FC validated the use of resting-state fMRI signals for probing neural intrinsic FC alterations in pathological deafferented cortex, and indicated that disrupted FC between mesoscale functionally highly related regions may contribute to the behavioral impairments.
Keywords: electrophysiology; fMRI; functional connectivity; nonhuman primate; spinal cord injury.
Copyright © 2017 the authors 0270-6474/17/3711192-12$15.00/0.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
