Anti-PD-1 Antibody Treatment Promotes Clearance of Persistent Cryptococcal Lung Infection in Mice
- PMID: 29038249
- PMCID: PMC5687305
- DOI: 10.4049/jimmunol.1700840
Anti-PD-1 Antibody Treatment Promotes Clearance of Persistent Cryptococcal Lung Infection in Mice
Abstract
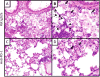
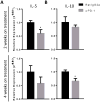
Activation of immunomodulatory pathways in response to invasive fungi can impair clearance and promote persistent infections. The programmed cell death protein-1 (PD-1) signaling pathway inhibits immune effector responses against tumors, and immune checkpoint inhibitors that block this pathway are being increasingly used as cancer therapy. The objective of this study was to investigate whether this pathway contributes to persistent fungal infection and to determine whether anti-PD-1 Ab treatment improves fungal clearance. Studies were performed using C57BL/6 mice infected with a moderately virulent strain of Cryptococcus neoformans (52D), which resulted in prolonged elevations in fungal burden and histopathologic evidence of chronic lung inflammation. Persistent infection was associated with increased and sustained expression of PD-1 on lung lymphocytes, including a mixed population of CD4+ T cells. In parallel, expression of the PD-1 ligands, PD-1 ligands 1 and 2, was similarly upregulated on specific subsets of resident and recruited lung dendritic cells and macrophages. Treatment of persistently infected mice for 4 wk by repetitive administration of neutralizing anti-PD-1 Ab significantly improved pulmonary fungal clearance. Treatment was well tolerated without evidence of morbidity. Immunophenotyping revealed that anti-PD-1 Ab treatment did not alter immune effector cell numbers or myeloid cell activation. Treatment did reduce gene expression of IL-5 and IL-10 by lung leukocytes and promoted sustained upregulation of OX40 by Th1 and Th17 cells. Collectively, this study demonstrates that PD-1 signaling promotes persistent cryptococcal lung infection and identifies this pathway as a potential target for novel immune-based treatments of chronic fungal disease.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures







Similar articles
-
Early or late IL-10 blockade enhances Th1 and Th17 effector responses and promotes fungal clearance in mice with cryptococcal lung infection.J Immunol. 2014 Oct 15;193(8):4107-16. doi: 10.4049/jimmunol.1400650. Epub 2014 Sep 15. J Immunol. 2014. PMID: 25225664 Free PMC article.
-
Autocrine IL-10 Signaling Promotes Dendritic Cell Type-2 Activation and Persistence of Murine Cryptococcal Lung Infection.J Immunol. 2018 Oct 1;201(7):2004-2015. doi: 10.4049/jimmunol.1800070. Epub 2018 Aug 10. J Immunol. 2018. PMID: 30097531 Free PMC article.
-
Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production.Infect Immun. 2014 Mar;82(3):937-48. doi: 10.1128/IAI.01477-13. Epub 2013 Dec 9. Infect Immun. 2014. PMID: 24324191 Free PMC article.
-
Cryptococcus neoformans and cryptococcosis.J Infect. 2000 Jul;41(1):12-7. doi: 10.1053/jinf.2000.0695. J Infect. 2000. PMID: 11041708 Review. No abstract available.
-
Are macrophages the heroes or villains during cryptococcosis?Fungal Genet Biol. 2019 Nov;132:103261. doi: 10.1016/j.fgb.2019.103261. Epub 2019 Aug 12. Fungal Genet Biol. 2019. PMID: 31415906 Review.
Cited by
-
Fungal infection risks associated with the use of cytokine antagonists and immune checkpoint inhibitors.Exp Biol Med (Maywood). 2020 Jul;245(13):1104-1114. doi: 10.1177/1535370220939862. Epub 2020 Jul 8. Exp Biol Med (Maywood). 2020. PMID: 32640893 Free PMC article. Review.
-
[Pneumocystis jirovecii Pneumonia in Patients with Lung Cancer: A Review].Zhongguo Fei Ai Za Zhi. 2022 Apr 20;25(4):272-277. doi: 10.3779/j.issn.1009-3419.2022.101.14. Epub 2022 Mar 28. Zhongguo Fei Ai Za Zhi. 2022. PMID: 35340199 Free PMC article. Review. Chinese.
-
Transcriptional profiling of a fungal granuloma reveals a low metabolic activity of Paracoccidioides brasiliensis yeasts and an actively regulated host immune response.Front Cell Infect Microbiol. 2023 Oct 5;13:1268959. doi: 10.3389/fcimb.2023.1268959. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37868350 Free PMC article.
-
Inbred Mouse Models in Cryptococcus neoformans Research.J Fungi (Basel). 2024 Jun 17;10(6):426. doi: 10.3390/jof10060426. J Fungi (Basel). 2024. PMID: 38921412 Free PMC article. Review.
-
Use of Clinical Isolates to Establish Criteria for a Mouse Model of Latent Cryptococcus neoformans Infection.Front Cell Infect Microbiol. 2022 Feb 2;11:804059. doi: 10.3389/fcimb.2021.804059. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35186781 Free PMC article.
References
-
- Li SS, Mody CH. Cryptococcus. Proceedings of the American Thoracic Society. 2010;7:186–196. - PubMed
-
- Bozzette SA, Larsen RA, Chiu J, Leal MA, Jacobsen J, Rothman P, Robinson P, Gilbert G, McCutchan JA, Tilles J, et al. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. The New England journal of medicine. 1991;324:580–584. - PubMed
-
- Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, Marriott D, Pfeiffer T, Parr D, Byth K. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2000;31:499–508. - PubMed
-
- Choi YH, Ngamskulrungroj P, Varma A, Sionov E, Hwang SM, Carriconde F, Meyer W, Litvintseva AP, Lee WG, Shin JH, Kim EC, Lee KW, Choi TY, Lee YS, Kwon-Chung KJ. Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the Republic of Korea. FEMS yeast research. 2010;10:769–778. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

