Stress Introduction Rate Alters the Benefit of AcrAB-TolC Efflux Pumps
- PMID: 29038251
- PMCID: PMC5717160
- DOI: 10.1128/JB.00525-17
Stress Introduction Rate Alters the Benefit of AcrAB-TolC Efflux Pumps
Abstract
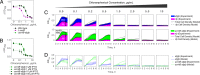
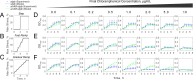
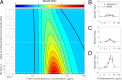
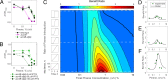
Stress tolerance studies are typically conducted in an all-or-none fashion. However, in realistic settings-such as in clinical or metabolic engineering applications-cells may encounter stresses at different rates. Therefore, how cells tolerate stress may depend on its rate of appearance. To address this, we studied how the rate of stress introduction affects bacterial stress tolerance by focusing on a key stress response mechanism. Efflux pumps, such as AcrAB-TolC of Escherichia coli, are membrane transporters well known for the ability to export a wide variety of substrates, including antibiotics, signaling molecules, and biofuels. Although efflux pumps improve stress tolerance, pump overexpression can result in a substantial fitness cost to the cells. We hypothesized that the ideal pump expression level would involve a rate-dependent trade-off between the benefit of pumps and the cost of their expression. To test this, we evaluated the benefit of the AcrAB-TolC pump under different rates of stress introduction, including a step, a fast ramp, and a gradual ramp. Using two chemically diverse stresses, the antibiotic chloramphenicol and the jet biofuel precursor pinene, we assessed the benefit provided by the pumps. A mathematical model describing these effects predicted the benefit as a function of the rate of stress introduction. Our findings demonstrate that as the rate of introduction is lowered, stress response mechanisms provide a disproportionate benefit to pump-containing strains, allowing cells to survive beyond the original inhibitory concentrations.IMPORTANCE Efflux pumps are ubiquitous in nature and provide stress tolerance in the cells of species ranging from bacteria to mammals. Understanding how pumps provide tolerance has far-reaching implications for diverse fields, from medicine to biotechnology. Here, we investigated how the rate of stressor appearance impacts tolerance. We focused on two distinct substrates of AcrAB-TolC efflux pumps, the antibiotic chloramphenicol and the biofuel precursor pinene. Interestingly, tolerance is highly dependent on the rate of stress introduction. Therefore, it is important to consider not only the total quantity of a stressor but also the rate at which it is applied. The implications of this work are significant because environments are rarely static; antibiotic concentrations change during dosing, and metabolic engineering processes change with time.
Keywords: antibiotics; biofuels; dynamic environment; efflux pumps; stress tolerance.
Copyright © 2017 American Society for Microbiology.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

