Fidaxomicin and OP-1118 Inhibit Clostridium difficile Toxin A- and B-Mediated Inflammatory Responses via Inhibition of NF-κB Activity
- PMID: 29038278
- PMCID: PMC5740352
- DOI: 10.1128/AAC.01513-17
Fidaxomicin and OP-1118 Inhibit Clostridium difficile Toxin A- and B-Mediated Inflammatory Responses via Inhibition of NF-κB Activity
Abstract
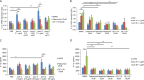
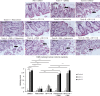
Clostridium difficile causes diarrhea and colitis by releasing toxin A and toxin B. In the human colon, both toxins cause intestinal inflammation and stimulate tumor necrosis factor alpha (TNF-α) expression via the activation of NF-κB. It is well established that the macrolide antibiotic fidaxomicin is associated with reduced relapses of C. difficile infection. We showed that fidaxomicin and its primary metabolite OP-1118 significantly inhibited toxin A-mediated intestinal inflammation in mice in vivo and toxin A-induced cell rounding in vitro We aim to determine whether fidaxomicin and OP-1118 possess anti-inflammatory effects against toxin A and toxin B in the human colon and examine the mechanism of this response. We used fresh human colonic explants, NCM460 human colonic epithelial cells, and RAW264.7 mouse macrophages to study the mechanism of the activity of fidaxomicin and OP-1118 against toxin A- and B-mediated cytokine expression and apoptosis. Fidaxomicin and OP-1118 dose-dependently inhibited toxin A- and B-induced TNF-α and interleukin-1β (IL-1β) mRNA expression and histological damage in human colonic explants. Fidaxomicin and OP-1118 inhibited toxin A-mediated NF-κB phosphorylation in human and mouse intestinal mucosae. Fidaxomicin and OP-1118 also inhibited toxin A-mediated NF-κB phosphorylation and TNF-α expression in macrophages, which was reversed by the NF-κB activator phorbol myristate acetate (PMA). Fidaxomicin and OP-1118 prevented toxin A- and B-mediated apoptosis in NCM460 cells, which was reversed by the addition of PMA. PMA reversed the cytoprotective effect of fidaxomicin and OP-1118 in toxin-exposed human colonic explants. Fidaxomicin and OP-1118 inhibit C. difficile toxin A- and B-mediated inflammatory responses, NF-κB phosphorylation, and tissue damage in the human colon.
Keywords: Clostridium difficile infection; antibiotics; signaling.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum.Antimicrob Agents Chemother. 2014 Aug;58(8):4642-50. doi: 10.1128/AAC.02783-14. Epub 2014 Jun 2. Antimicrob Agents Chemother. 2014. PMID: 24890583 Free PMC article.
-
Human monoclonal antibodies against Clostridium difficile toxins A and B inhibit inflammatory and histologic responses to the toxins in human colon and peripheral blood monocytes.Antimicrob Agents Chemother. 2013 Jul;57(7):3214-23. doi: 10.1128/AAC.02633-12. Epub 2013 Apr 29. Antimicrob Agents Chemother. 2013. PMID: 23629713 Free PMC article.
-
Clostridium difficile toxin B induces colonic inflammation through the TRIM46/DUSP1/MAPKs and NF-κB signalling pathway.Artif Cells Nanomed Biotechnol. 2020 Dec;48(1):452-462. doi: 10.1080/21691401.2019.1709856. Artif Cells Nanomed Biotechnol. 2020. Retraction in: Artif Cells Nanomed Biotechnol. 2023 Dec;51(1):241. doi: 10.1080/21691401.2023.2208911. PMID: 31918570 Retracted.
-
Effects of Clostridium difficile toxins on epithelial cell barrier.Ann N Y Acad Sci. 2000;915:347-56. doi: 10.1111/j.1749-6632.2000.tb05263.x. Ann N Y Acad Sci. 2000. PMID: 11193598 Review.
-
Biological Mechanisms of Polyphenols against Clostridium Difficile: A Systematic Review.Infect Disord Drug Targets. 2025;25(3):e18715265313944. doi: 10.2174/0118715265313944240726115600. Infect Disord Drug Targets. 2025. PMID: 39234903
Cited by
-
Clinical and microbiological analysis of risk factors for breakthrough bloodstream infection during Tigecycline Therapy.Sci Rep. 2025 Feb 4;15(1):4266. doi: 10.1038/s41598-025-88048-7. Sci Rep. 2025. PMID: 39905181 Free PMC article.
-
Exploring the Toxin-Mediated Mechanisms in Clostridioides difficile Infection.Microorganisms. 2024 May 16;12(5):1004. doi: 10.3390/microorganisms12051004. Microorganisms. 2024. PMID: 38792835 Free PMC article. Review.
-
Synthetic niclosamide-loaded controlled-release nanospheres with high solubility and stability exerting multiple effects against Clostridioides difficile.Front Microbiol. 2025 Jul 30;16:1617631. doi: 10.3389/fmicb.2025.1617631. eCollection 2025. Front Microbiol. 2025. PMID: 40809058 Free PMC article.
-
Fidaxomicin for the Treatment of Clostridioides difficile Infection in Adult Patients: An Update on Results from Randomized Controlled Trials.Antibiotics (Basel). 2022 Oct 6;11(10):1365. doi: 10.3390/antibiotics11101365. Antibiotics (Basel). 2022. PMID: 36290023 Free PMC article. Review.
-
Ceragenin CSA13 Reduces Clostridium difficile Infection in Mice by Modulating the Intestinal Microbiome and Metabolites.Gastroenterology. 2018 May;154(6):1737-1750. doi: 10.1053/j.gastro.2018.01.026. Epub 2018 Jan 31. Gastroenterology. 2018. PMID: 29360463 Free PMC article.
References
-
- Koon HW, Shih DQ, Hing TC, Yoo JH, Ho S, Chen X, Kelly CP, Targan SR, Pothoulakis C. 2013. Human monoclonal antibodies against Clostridium difficile toxins A and B inhibit inflammatory and histologic responses to the toxins in human colon and peripheral blood monocytes. Antimicrob Agents Chemother 57:3214–3223. doi:10.1128/AAC.02633-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

