Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy
- PMID: 29038282
- PMCID: PMC5740314
- DOI: 10.1128/AAC.01710-17
Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy
Abstract
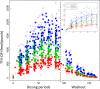
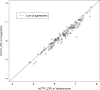
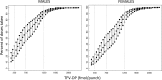
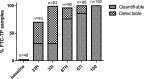
Studies of daily emtricitabine-tenofovir disoproxil fumarate (FTC-TDF) for HIV preexposure prophylaxis (PrEP) in men who have sex with men (MSM) modeled intracellular tenofovir-diphosphate (TFV-DP) in dried blood spots (DBS) to assess adherence and corresponding PrEP outcomes. We conducted a prospective, randomized, crossover pharmacokinetic study of TFV-DP in DBS during 33%, 67%, or 100% of daily dosing under directly observed therapy (DOT). Participants were assigned to two 12-week dosing regimens, separated by a 12-week washout. Forty-eight adults (25 women) from Denver and San Francisco were included. TFV-DP exhibited a median half-life of 17 days, reaching steady state in 8 weeks. TFV-DP was dose proportional with mean (SD) steady-state concentrations of 530 (159), 997 (267), and 1,605 (405) fmol/punch for the 33%, 67%, and 100% arms, respectively. Prior work in MSM demonstrated clinically meaningful TFV-DP thresholds of 350, 700, and 1,250 fmol/punch, which were estimated 25th percentiles for 2, 4, and 7 doses/week. In the present study, corresponding TFV-DP was within 3% of the prior estimates, and subgroups by site, race, and sex were within 14% of prior estimates, although males had 17.6% (95% confidence intervals [CIs], 6.5, 27.4%) lower TFV-DP than females. The thresholds of 350, 700, and 1,250 fmol/punch were achieved by 75% of men taking ≥1.2, 3.2, and 6 doses/week and 75% of women taking ≥0.6, 2.0, and 5.3 doses/week, indicating that lower dosing reached these thresholds for both sexes. In conclusion, TFV-DP arising from DOT was similar to previous estimates and is useful for interpreting PrEP adherence and study outcomes. (This study has been registered at ClinicalTrials.gov under identifier NCT02022657.).
Keywords: HIV; adherence; intracellular; nucleoside analog; pharmacokinetics; preexposure prophylaxis.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi: 10.1056/NEJMoa1011205. - DOI - PMC - PubMed
-
- Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Masse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM. 2015. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 372:509–518. doi: 10.1056/NEJMoa1402269. - DOI - PMC - PubMed
-
- Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 367:411–422. doi: 10.1056/NEJMoa1202614. - DOI - PMC - PubMed
-
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367:399–410. doi: 10.1056/NEJMoa1108524. - DOI - PMC - PubMed
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367:423–434. doi: 10.1056/NEJMoa1110711. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

