Cystine uptake through the cystine/glutamate antiporter xCT triggers glioblastoma cell death under glucose deprivation
- PMID: 29038291
- PMCID: PMC5712613
- DOI: 10.1074/jbc.M117.814392
Cystine uptake through the cystine/glutamate antiporter xCT triggers glioblastoma cell death under glucose deprivation
Abstract
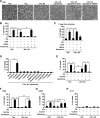
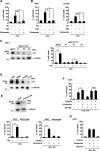
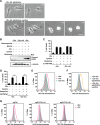
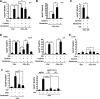
Oncogenic signaling in cancer cells alters glucose uptake and utilization to supply sufficient energy and biosynthetic intermediates for survival and sustained proliferation. Oncogenic signaling also prevents oxidative stress and cell death caused by increased production of reactive oxygen species. However, elevated glucose metabolism in cancer cells, especially in glioblastoma, results in the cells becoming sensitive to glucose deprivation (i.e. in high glucose dependence), which rapidly induces cell death. However, the precise mechanism of this type of cell death remains unknown. Here, we report that glucose deprivation alone does not trigger glioblastoma cell death. We found that, for cell death to occur in glucose-deprived glioblastoma cells, cystine and glutamine also need to be present in culture media. We observed that cystine uptake through the cystine/glutamate antiporter xCT under glucose deprivation rapidly induces NADPH depletion, reactive oxygen species accumulation, and cell death. We conclude that although cystine uptake is crucial for production of antioxidant glutathione in cancer cells its transport through xCT also induces oxidative stress and cell death in glucose-deprived glioblastoma cells. Combining inhibitors targeting cancer-specific glucose metabolism with cystine and glutamine treatment may offer a therapeutic approach for glioblastoma tumors exhibiting high xCT expression.
Keywords: amino acid transport; cell death; cystine; glioblastoma; glucose; reactive oxygen species (ROS).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation.Genes Dis. 2020 Nov 25;8(6):731-745. doi: 10.1016/j.gendis.2020.11.010. eCollection 2021 Nov. Genes Dis. 2020. PMID: 34522704 Free PMC article. Review.
-
A synthetic lethal drug combination mimics glucose deprivation-induced cancer cell death in the presence of glucose.J Biol Chem. 2020 Jan 31;295(5):1350-1365. doi: 10.1074/jbc.RA119.011471. Epub 2019 Dec 30. J Biol Chem. 2020. PMID: 31914417 Free PMC article.
-
High cell density increases glioblastoma cell viability under glucose deprivation via degradation of the cystine/glutamate transporter xCT (SLC7A11).J Biol Chem. 2020 May 15;295(20):6936-6945. doi: 10.1074/jbc.RA119.012213. Epub 2020 Apr 7. J Biol Chem. 2020. PMID: 32265299 Free PMC article.
-
The cystine/glutamate antiporter xCT is a key regulator of EphA2 S897 phosphorylation under glucose-limited conditions.Cell Signal. 2019 Oct;62:109329. doi: 10.1016/j.cellsig.2019.05.014. Epub 2019 May 29. Cell Signal. 2019. PMID: 31152846
-
Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target.CNS Neurol Disord Drug Targets. 2010 Jul;9(3):373-82. doi: 10.2174/187152710791292567. CNS Neurol Disord Drug Targets. 2010. PMID: 20053169 Review.
Cited by
-
A xCT role in tumour-associated ferroptosis shed light on novel therapeutic options.Explor Target Antitumor Ther. 2022;3(5):570-581. doi: 10.37349/etat.2022.00101. Epub 2022 Oct 25. Explor Target Antitumor Ther. 2022. PMID: 36338517 Free PMC article.
-
Ferroptosis Involvement in Glioblastoma Treatment.Medicina (Kaunas). 2022 Feb 20;58(2):319. doi: 10.3390/medicina58020319. Medicina (Kaunas). 2022. PMID: 35208642 Free PMC article. Review.
-
Metabolic Remodeling in Glioma Immune Microenvironment: Intercellular Interactions Distinct From Peripheral Tumors.Front Cell Dev Biol. 2021 Jun 11;9:693215. doi: 10.3389/fcell.2021.693215. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34211978 Free PMC article. Review.
-
NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation.Genes Dis. 2020 Nov 25;8(6):731-745. doi: 10.1016/j.gendis.2020.11.010. eCollection 2021 Nov. Genes Dis. 2020. PMID: 34522704 Free PMC article. Review.
-
Molecular map of disulfidptosis-related genes in lung adenocarcinoma: the perspective toward immune microenvironment and prognosis.Clin Epigenetics. 2024 Feb 11;16(1):26. doi: 10.1186/s13148-024-01632-y. Clin Epigenetics. 2024. PMID: 38342890 Free PMC article.
References
-
- Hanahan D., and Weinberg R. A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 - PubMed
-
- Furnari F. B., Fenton T., Bachoo R. M., Mukasa A., Stommel J. M., Stegh A., Hahn W. C., Ligon K. L., Louis D. N., Brennan C., Chin L., DePinho R. A., and Cavenee W. K. (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 21, 2683–2710 - PubMed
-
- Elstrom R. L., Bauer D. E., Buzzai M., Karnauskas R., Harris M. H., Plas D. R., Zhuang H., Cinalli R. M., Alavi A., Rudin C. M., and Thompson C. B. (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64, 3892–3899 - PubMed
-
- Jelluma N., Yang X., Stokoe D., Evan G. I., Dansen T. B., and Haas-Kogan D. A. (2006) Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal astrocytes. Mol. Cancer Res. 4, 319–330 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

