The NSAID allosteric site of human cytosolic sulfotransferases
- PMID: 29038294
- PMCID: PMC5724015
- DOI: 10.1074/jbc.M117.817387
The NSAID allosteric site of human cytosolic sulfotransferases
Abstract
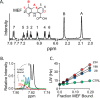
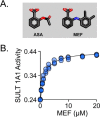
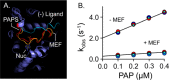
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most commonly prescribed drugs worldwide-more than 111 million prescriptions were written in the United States in 2014. NSAIDs allosterically inhibit cytosolic sulfotransferases (SULTs) with high specificity and therapeutically relevant affinities. This study focuses on the interactions of SULT1A1 and mefenamic acid (MEF)-a potent, highly specific NSAID inhibitor of 1A1. Here, the first structure of an NSAID allosteric site-the MEF-binding site of SULT1A1-is determined using spin-label triangulation NMR. The structure is confirmed by site-directed mutagenesis and provides a molecular framework for understanding NSAID binding and isoform specificity. The mechanism of NSAID inhibition is explored using molecular dynamics and equilibrium and pre-steady-state ligand-binding studies. MEF inhibits SULT1A1 turnover through an indirect (helix-mediated) stabilization of the closed form of the active-site cap of the enzyme, which traps the nucleotide and slows its release. Using the NSAID-binding site structure of SULT1A1 as a comparative model, it appears that 11 of the 13 human SULT isoforms harbor an NSAID-binding site. We hypothesize that these sites evolved to enable SULT isoforms to respond to metabolites that lie within their metabolic domains. Finally, the NSAID-binding site structure offers a template for developing isozyme-specific allosteric inhibitors that can be used to regulate specific areas of sulfuryl-transfer metabolism.
Keywords: NSAID; acetylsalicylic; allosteric regulation; kinetics; mefenamic; nuclear magnetic resonance (NMR); spin label; structural model; sulfotransferase.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Campbell C. L., Smyth S., Montalescot G., and Steinhubl S. R. (2007) Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 297, 2018–2024 - PubMed
-
- Strauch K. A. (2011) Invisible pollution: the impact of pharmaceuticals in the water supply. AAOHN J. 59, 525–532 - PubMed
-
- Fuster V., and Sweeny J. M. (2011) Aspirin: a historical and contemporary therapeutic overview. Circulation 123, 768–778 - PubMed
-
- Mitchell J. A., and Warner T. D. (2006) COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat. Rev. Drug Discov. 5, 75–86 - PubMed
-
- Mora S., and Manson J. E. (2016) Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern. Med. 176, 1195–1204 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

