Characterization of aromatic residue-controlled protein retention in the endoplasmic reticulum of Saccharomyces cerevisiae
- PMID: 29038295
- PMCID: PMC5733606
- DOI: 10.1074/jbc.M117.812107
Characterization of aromatic residue-controlled protein retention in the endoplasmic reticulum of Saccharomyces cerevisiae
Abstract
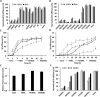
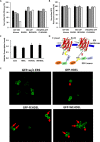
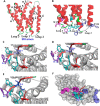
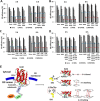
An endoplasmic reticulum (ER) retention sequence (ERS) is a characteristic short sequence that mediates protein retention in the ER of eukaryotic cells. However, little is known about the detailed molecular mechanism involved in ERS-mediated protein ER retention. Using a new surface display-based fluorescence technique that effectively quantifies ERS-promoted protein ER retention within Saccharomyces cerevisiae cells, we performed comprehensive ERS analyses. We found that the length, type of amino acid residue, and additional residues at positions -5 and -6 of the C-terminal HDEL motif all determined the retention of ERS in the yeast ER. Moreover, the biochemical results guided by structure simulation revealed that aromatic residues (Phe-54, Trp-56, and other aromatic residues facing the ER lumen) in both the ERS (at positions -6 and -4) and its receptor, Erd2, jointly determined their interaction with each other. Our studies also revealed that this aromatic residue interaction might lead to the discriminative recognition of HDEL or KDEL as ERS in yeast or human cells, respectively. Our findings expand the understanding of ERS-mediated residence of proteins in the ER and may guide future research into protein folding, modification, and translocation affected by ER retention.
Keywords: ER retention sequence; Erd2; endoplasmic reticulum (ER); flow cytometry; fluorescence; protein ER retention; protein sorting; protein trafficking (Golgi); surface display; yeast.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
This work was supported by Grant 31540068 from the National Natural Science Foundation of China (to L. Y.) and Grant 2014AA022203C from the Ministry of Science and Technology of China (to G. Z.). The authors declared that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Changing the specificity of the sorting receptor for luminal endoplasmic reticulum proteins.J Mol Biol. 1992 Mar 5;224(1):1-5. doi: 10.1016/0022-2836(92)90571-z. J Mol Biol. 1992. PMID: 1312604
-
ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway.Cell. 1990 Jun 29;61(7):1349-57. doi: 10.1016/0092-8674(90)90698-e. Cell. 1990. PMID: 2194670
-
Morphological and functional association of Sec22b/ERS-24 with the pre-Golgi intermediate compartment.Mol Biol Cell. 1999 Feb;10(2):435-53. doi: 10.1091/mbc.10.2.435. Mol Biol Cell. 1999. PMID: 9950687 Free PMC article.
-
Mechanism and engineering of endoplasmic reticulum-localized membrane protein folding in Saccharomyces cerevisiae.Metab Eng. 2025 Jul;90:43-56. doi: 10.1016/j.ymben.2025.03.006. Epub 2025 Mar 8. Metab Eng. 2025. PMID: 40064436 Review.
-
The concept of translocational regulation.J Cell Biol. 2008 Jul 28;182(2):225-32. doi: 10.1083/jcb.200804157. Epub 2008 Jul 21. J Cell Biol. 2008. PMID: 18644895 Free PMC article. Review.
Cited by
-
PERRC: Protease Engineering with Reactant Residence Time Control.bioRxiv [Preprint]. 2025 Mar 4:2025.03.02.641063. doi: 10.1101/2025.03.02.641063. bioRxiv. 2025. Update in: ACS Synth Biol. 2025 Jun 20;14(6):2241-2253. doi: 10.1021/acssynbio.5c00154. PMID: 40093119 Free PMC article. Updated. Preprint.
-
Exploration of the Key Pathways and Genes Involved in Osteoarthritis Genesis: Evidence from Multiple Platforms and Real-World Validation.J Inflamm Res. 2024 Dec 4;17:10223-10237. doi: 10.2147/JIR.S488935. eCollection 2024. J Inflamm Res. 2024. PMID: 39649419 Free PMC article.
-
PERRC: Protease Engineering with Reactant Residence Time Control.ACS Synth Biol. 2025 Jun 20;14(6):2241-2253. doi: 10.1021/acssynbio.5c00154. Epub 2025 May 19. ACS Synth Biol. 2025. PMID: 40388903 Free PMC article.
-
A convenient broad-host counterselectable system endowing rapid genetic manipulations of Kluyveromyces lactis and other yeast species.Microb Cell Fact. 2024 Jul 26;23(1):212. doi: 10.1186/s12934-024-02488-w. Microb Cell Fact. 2024. PMID: 39061053 Free PMC article.
-
KDEL Receptors Are Differentially Regulated to Maintain the ER Proteome under Calcium Deficiency.Cell Rep. 2018 Nov 13;25(7):1829-1840.e6. doi: 10.1016/j.celrep.2018.10.055. Cell Rep. 2018. PMID: 30428351 Free PMC article.
References
-
- Braakman I., and Bulleid N. J. (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 80, 71–99 - PubMed
-
- Gregersen N., Bross P., Vang S., and Christensen J. H. (2006) Protein misfolding and human disease. Annu. Rev. Genomics Hum. Genet. 7, 103–124 - PubMed
-
- Munro S., and Pelham H. R. (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48, 899–907 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

