Characterization of aromatic residue-controlled protein retention in the endoplasmic reticulum of Saccharomyces cerevisiae
- PMID: 29038295
- PMCID: PMC5733606
- DOI: 10.1074/jbc.M117.812107
Characterization of aromatic residue-controlled protein retention in the endoplasmic reticulum of Saccharomyces cerevisiae
Abstract
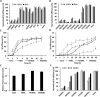
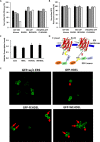
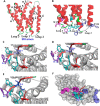
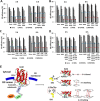
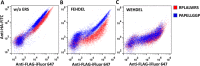
An endoplasmic reticulum (ER) retention sequence (ERS) is a characteristic short sequence that mediates protein retention in the ER of eukaryotic cells. However, little is known about the detailed molecular mechanism involved in ERS-mediated protein ER retention. Using a new surface display-based fluorescence technique that effectively quantifies ERS-promoted protein ER retention within Saccharomyces cerevisiae cells, we performed comprehensive ERS analyses. We found that the length, type of amino acid residue, and additional residues at positions -5 and -6 of the C-terminal HDEL motif all determined the retention of ERS in the yeast ER. Moreover, the biochemical results guided by structure simulation revealed that aromatic residues (Phe-54, Trp-56, and other aromatic residues facing the ER lumen) in both the ERS (at positions -6 and -4) and its receptor, Erd2, jointly determined their interaction with each other. Our studies also revealed that this aromatic residue interaction might lead to the discriminative recognition of HDEL or KDEL as ERS in yeast or human cells, respectively. Our findings expand the understanding of ERS-mediated residence of proteins in the ER and may guide future research into protein folding, modification, and translocation affected by ER retention.
Keywords: ER retention sequence; Erd2; endoplasmic reticulum (ER); flow cytometry; fluorescence; protein ER retention; protein sorting; protein trafficking (Golgi); surface display; yeast.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
This work was supported by Grant 31540068 from the National Natural Science Foundation of China (to L. Y.) and Grant 2014AA022203C from the Ministry of Science and Technology of China (to G. Z.). The authors declared that they have no conflicts of interest with the contents of this article
Figures


References
-
- Braakman I., and Bulleid N. J. (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 80, 71–99 - PubMed
-
- Gregersen N., Bross P., Vang S., and Christensen J. H. (2006) Protein misfolding and human disease. Annu. Rev. Genomics Hum. Genet. 7, 103–124 - PubMed
-
- Munro S., and Pelham H. R. (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48, 899–907 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

