Local production of neurostradiol affects gonadotropin-releasing hormone (GnRH) secretion at mid-gestation in Lagostomus maximus (Rodentia, Caviomorpha)
- PMID: 29038356
- PMCID: PMC5641931
- DOI: 10.14814/phy2.13439
Local production of neurostradiol affects gonadotropin-releasing hormone (GnRH) secretion at mid-gestation in Lagostomus maximus (Rodentia, Caviomorpha)
Abstract
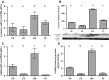
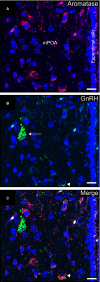
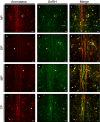
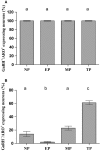
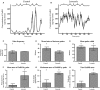
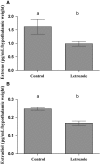
Females of the South American plains vizcacha, Lagostomus maximus, show peculiar reproductive features such as massive polyovulation up to 800 oocytes per estrous cycle and an ovulatory process around mid-gestation arising from the reactivation of the hypothalamic-hypophyseal-ovary (H.H.O.) axis. Estradiol (E2) regulates gonadotropin-releasing hormone (GnRH) expression. Biosynthesis of estrogens results from the aromatization of androgens by aromatase, which mainly occurs in the gonads, but has also been described in the hypothalamus. The recently described correlation between GnRH and ERα expression patterns in the hypothalamus of the vizcacha during pregnancy, with coexpression in the same neurons of the medial preoptic area, suggests that hypothalamic synthesis of E2 may affect GnRH neurons and contribute with systemic E2 to modulate GnRH delivery during the gestation. To elucidate this hypothesis, hypothalamic expression and the action of aromatase on GnRH release were evaluated in female vizcachas throughout pregnancy. Aromatase and GnRH expression was increased significantly in mid-pregnant and term-pregnant vizcachas compared to early-pregnant and nonpregnant females. In addition, aromatase and GnRH were colocalized in neurons of the medial preoptic area of the hypothalamus throughout gestation. The blockage of the negative feedback of E2 induced by the inhibition of aromatase resulted in a significant increment of GnRH-secreted mass by hypothalamic explants. E2 produced in the same neurons as GnRH may drive intracellular E2 to higher levels than those obtained from systemic circulation alone. This may trigger for a prompt GnRH availability enabling H.H.O. activity at mid-gestation with ovulation and formation of accessory corpora lutea with steroidogenic activity that produce the necessary progesterone to maintain gestation to term and guarantee the reproductive success.
Keywords: LH; Lagostomus maximus; Estradiol; GnRH; reproduction.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures
Similar articles
-
ERα and GnRH co-localize in the hypothalamic neurons of the South American plains vizcacha, Lagostomus maximus (Rodentia, Caviomorpha).J Mol Histol. 2017 Jun;48(3):259-273. doi: 10.1007/s10735-017-9715-6. Epub 2017 Mar 19. J Mol Histol. 2017. PMID: 28317066
-
Variation in progesterone receptors and GnRH expression in the hypothalamus of the pregnant South American plains vizcacha, Lagostomus maximus (Mammalia, Rodentia).Biol Reprod. 2013 Nov 14;89(5):115. doi: 10.1095/biolreprod.113.107995. Print 2013 Nov. Biol Reprod. 2013. PMID: 24089203
-
Pituitary estrogen receptor alpha is involved in luteinizing hormone pulsatility at mid-gestation in the South American plains vizcacha, Lagostomus maximus (Rodentia, Caviomorpha).Gen Comp Endocrinol. 2019 Mar 1;273:40-51. doi: 10.1016/j.ygcen.2018.04.001. Epub 2018 Apr 12. Gen Comp Endocrinol. 2019. PMID: 29656043
-
Neuroestradiol in regulation of GnRH release.Horm Behav. 2018 Aug;104:138-145. doi: 10.1016/j.yhbeh.2018.04.003. Epub 2018 Apr 19. Horm Behav. 2018. PMID: 29626484 Free PMC article. Review.
-
Neuroendocrine signals in the regulation of gonadotropin-releasing hormone secretion.Chin J Physiol. 1997 Dec 31;40(4):181-96. Chin J Physiol. 1997. PMID: 9551247 Review.
Cited by
-
Structural organization, GABAergic and tyrosine hydroxylase expression in the striatum and globus pallidus of the South American plains vizcacha, Lagostomus maximus (Rodentia, Caviomorpha).J Mol Histol. 2019 Dec;50(6):515-531. doi: 10.1007/s10735-019-09845-9. Epub 2019 Sep 12. J Mol Histol. 2019. PMID: 31515635
-
Melatonin is involved in the modulation of the hypothalamic and pituitary activity in the South American plains vizcacha, Lagostomus maximus.J Comp Physiol B. 2022 Jan;192(1):141-159. doi: 10.1007/s00360-021-01405-6. Epub 2021 Aug 30. J Comp Physiol B. 2022. PMID: 34459966
References
-
- Abraham, G. E. , Odell W. D., Swerdloff R. S., and Hopper K.. 1972. Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17‐hydroxyprogesterone and estradiol‐17β during the menstrual cycle. J. Clin. Endocrinol. Metab. 34:312–318. - PubMed
-
- Abrahám, I. M. , Todman M. G., Korach K. S., and Herbison A. E.. 2004. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinol. 145:3055–3061. - PubMed
-
- Balthazart, J. , Baillien M., Charlier T. D., and Ball G. F.. 2003. Calcium dependent phosphorylation processes control brain aromatase in quail. Eur. J. Neurosci. 17:1591–1606. - PubMed
-
- Boukhliq, R. , Goodman R. L., Berriman S. J., Adrian B., and Lehman M. N.. 1999. A subset of gonadotropin‐releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinol. 140:5929–5936. - PubMed
-
- Bradford, M. M. 1976. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 72:248–254. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

