One Dimensional Coordination Polymer of Zn(II) for Developing Multifunctional Nanoparticles
- PMID: 29038429
- PMCID: PMC5643562
- DOI: 10.1038/s41598-017-12980-6
One Dimensional Coordination Polymer of Zn(II) for Developing Multifunctional Nanoparticles
Abstract
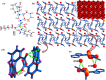
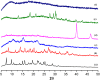
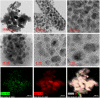
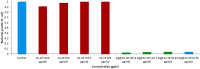
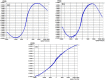
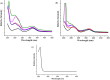
A variety of nanoparticles (NPs) including Ag, Au, Pd, Cr and mixed Cu/Fe have been synthesized in a non-activated (without solvent removal) one dimensional coordination polymer (CP) of Zn(II) via two different mechanisms, acid formation and redox activity of the framework. Main driving force to grow these NPs within the cavities of CP is the presence of free oxygens of one of the monodentate carboxylate groups of BDC ligand. These free oxygens act as anchoring sites for the metal ions of the metal precursors. Chemical and physical characteristics of the NPs within the framework have been evaluated by the high resolution transmission electron microscopic (HRTEM) images. Excluding Ag(0) and Pd(0) other NPs are present as combinations of their elemental as well as oxide forms (Au/Au2O3, Cr/Cr2O3/CrO2 and Cu/Cu2O, Fe/FeO). Synthesized Ag NPs within the framework show remarkable antibacterial efficacy at extremely low concentrations. Ag, Au and Cu/Fe NPs show ferromagnetic properties within the framework at room temperature. This polymer has potential to sequester highly toxic Cr(VI) to non toxic Cr(0), Cr(III) and Cr(IV) species.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Synthesis of non-toxic As and Cr nanoparticles through redox activity of highly flexible layered coordination polymer of Ni(II).Nanotechnology. 2018 Mar 9;29(10):105601. doi: 10.1088/1361-6528/aa9fa2. Nanotechnology. 2018. PMID: 29210670
-
Ligational behaviour of lomefloxacin drug towards Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Th(IV) and UO(2)(VI) ions: synthesis, structural characterization and biological activity studies.Spectrochim Acta A Mol Biomol Spectrosc. 2011 Nov;82(1):8-19. doi: 10.1016/j.saa.2011.05.089. Epub 2011 Jun 1. Spectrochim Acta A Mol Biomol Spectrosc. 2011. PMID: 21855402
-
Synthesis and redox activity of "clicked" triazolylbiferrocenyl polymers, network encapsulation of gold and silver nanoparticles and anion sensing.Inorg Chem. 2015 Mar 2;54(5):2284-99. doi: 10.1021/ic5028916. Epub 2015 Feb 13. Inorg Chem. 2015. PMID: 25676664
-
High-nuclearity metal-cyanide clusters: synthesis, magnetic properties, and inclusion behavior of open-cage species incorporating [(tach)M(CN)3] (M = Cr, Fe, Co) complexes.Inorg Chem. 2003 Mar 10;42(5):1403-19. doi: 10.1021/ic026065f. Inorg Chem. 2003. PMID: 12611505
-
Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions.Environ Int. 2011 Aug;37(6):1083-97. doi: 10.1016/j.envint.2011.03.009. Epub 2011 Apr 6. Environ Int. 2011. PMID: 21474182 Review.
Cited by
-
A Zn(II) Coordination Polymer for Fluorescent Turn-Off Selective Sensing of Heavy Metal Cation and Toxic Inorganic Anions.Molecules. 2024 Jun 20;29(12):2943. doi: 10.3390/molecules29122943. Molecules. 2024. PMID: 38931007 Free PMC article.
-
Zinc(II) Carboxylate Coordination Polymers with Versatile Applications.Molecules. 2023 Jan 23;28(3):1132. doi: 10.3390/molecules28031132. Molecules. 2023. PMID: 36770799 Free PMC article. Review.
-
Crystal Structures, Hirshfeld Surfaces, and Thermal Study of Isostructural Polymeric Ladders of La(III) and Sm(III) Coordination Compounds with 4,4'-Bipyridine and Dibromoacetates.Materials (Basel). 2020 Sep 25;13(19):4274. doi: 10.3390/ma13194274. Materials (Basel). 2020. PMID: 32993002 Free PMC article.
References
-
- Kitagawa, S., Kitaura, R. & Noro, S.-i. Functional Porous Coordination Polymers. Angew. Chem., Int. Ed. 43, 2334–2375 (2004). - PubMed
-
- Férey, G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 37, 191–214 (2008). - PubMed
-
- Kepert, C. J. Advanced functional properties in nanoporous coordination framework materials. Chem. Commun. 695–700 (2006). - PubMed
-
- Lue J-T. A review of characterization and physical property studies of metallic nanoparticles. J. Phys. Chem. Solids. 2001;62:1599–1612. doi: 10.1016/S0022-3697(01)00099-3. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

