Myoblasts and macrophages are required for therapeutic morpholino antisense oligonucleotide delivery to dystrophic muscle
- PMID: 29038471
- PMCID: PMC5643396
- DOI: 10.1038/s41467-017-00924-7
Myoblasts and macrophages are required for therapeutic morpholino antisense oligonucleotide delivery to dystrophic muscle
Erratum in
-
Author Correction: Myoblasts and macrophages are required for therapeutic morpholino antisense oligonucleotide delivery to dystrophic muscle.Nat Commun. 2018 Jan 15;9(1):208. doi: 10.1038/s41467-017-02206-8. Nat Commun. 2018. PMID: 29335405 Free PMC article.
-
Author Correction: Myoblasts and macrophages are required for therapeutic morpholino antisense oligonucleotide delivery to dystrophic muscle.Nat Commun. 2018 Mar 23;9(1):1256. doi: 10.1038/s41467-018-03709-8. Nat Commun. 2018. PMID: 29572439 Free PMC article.
Abstract
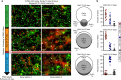
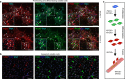
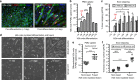
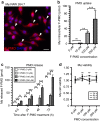
Exon skipping is a promising therapeutic strategy for Duchenne muscular dystrophy (DMD), employing morpholino antisense oligonucleotides (PMO-AO) to exclude disruptive exons from the mutant DMD transcript and elicit production of truncated dystrophin protein. Clinical trials for PMO show variable and sporadic dystrophin rescue. Here, we show that robust PMO uptake and efficient production of dystrophin following PMO administration coincide with areas of myofiber regeneration and inflammation. PMO localization is sustained in inflammatory foci where it enters macrophages, actively differentiating myoblasts and newly forming myotubes. We conclude that efficient PMO delivery into muscle requires two concomitant events: first, accumulation and retention of PMO within inflammatory foci associated with dystrophic lesions, and second, fusion of PMO-loaded myoblasts into repairing myofibers. Identification of these factors accounts for the variability in clinical trials and suggests strategies to improve this therapeutic approach to DMD.Exon skipping is a strategy for the treatment of Duchenne muscular dystrophy, but has variable efficacy. Here, the authors show that dystrophin restoration occurs preferentially in areas of myofiber regeneration, where antisense oligonucleotides are stored in macrophages and delivered to myoblasts and newly formed myotubes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
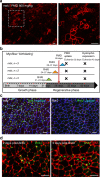
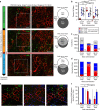

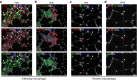
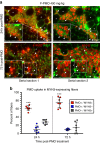
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

