Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury
- PMID: 29038483
- PMCID: PMC5643511
- DOI: 10.1038/s41598-017-13581-z
Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury
Abstract
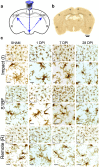
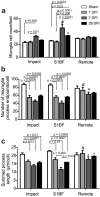
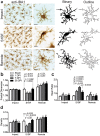
Determining regions of altered brain physiology after diffuse brain injury is challenging. Microglia, brain immune cells with ramified and dynamically moving processes, constantly surveil the parenchyma for dysfunction which, when present, results in a changed morphology. Our purpose was to define the spatiotemporal changes in microglia morphology over 28 days following rat midline fluid percussion injury (mFPI) as a first step in exploiting microglia morphology to reflect altered brain physiology. Microglia morphology was quantified from histological sections using Image J skeleton and fractal analysis procedures at three time points and in three regions post-mFPI: impact site, primary somatosensory cortex barrel field (S1BF), and a remote region. Microglia ramification (process length/cell and endpoints/cell) decreased in the impact and S1BF but not the remote region (p < 0.05). Microglia complexity was decreased in the S1BF (p = 0.003) and increased in the remote region (p < 0.02). Rod-shaped microglia were present in the S1BF and had a 1.8:1.0 length:width ratio. An in-depth quantitative morphologic analysis revealed diverse and widespread changes to microglia morphology in the cortex post-mFPI. Due to their close link to neuronal function, changes in microglia morphology, summarized in this study, likely reflect altered physiology with diverse and widespread impact on neuronal and circuit function.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Centers for Disease Control and Prevention. Report to Congress on Traumatic Brain Injury In the United States: Epidemiology and Rehabilitation. (National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention. Atlanta, GA) (2015).
-
- Leo, P. & McCrea, M. Frontiers in Neuroscience: Epidemiology. In Translational Research in Traumatic Brain Injury (eds Laskowitz, D. & Grant, G.) (CRC Press/Taylor and Francis Group, Boca Raton (FL), 2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

