Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2
- PMID: 29038491
- PMCID: PMC5643516
- DOI: 10.1038/s41467-017-01035-z
Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2
Abstract
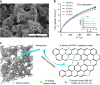
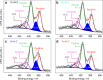
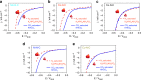
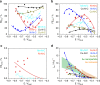
Direct electrochemical reduction of CO2 to fuels and chemicals using renewable electricity has attracted significant attention partly due to the fundamental challenges related to reactivity and selectivity, and partly due to its importance for industrial CO2-consuming gas diffusion cathodes. Here, we present advances in the understanding of trends in the CO2 to CO electrocatalysis of metal- and nitrogen-doped porous carbons containing catalytically active M-N x moieties (M = Mn, Fe, Co, Ni, Cu). We investigate their intrinsic catalytic reactivity, CO turnover frequencies, CO faradaic efficiencies and demonstrate that Fe-N-C and especially Ni-N-C catalysts rival Au- and Ag-based catalysts. We model the catalytically active M-N x moieties using density functional theory and correlate the theoretical binding energies with the experiments to give reactivity-selectivity descriptors. This gives an atomic-scale mechanistic understanding of potential-dependent CO and hydrocarbon selectivity from the M-N x moieties and it provides predictive guidelines for the rational design of selective carbon-based CO2 reduction catalysts.Inexpensive and selective electrocatalysts for CO2 reduction hold promise for sustainable fuel production. Here, the authors report N-coordinated, non-noble metal-doped porous carbons as efficient and selective electrocatalysts for CO2 to CO conversion.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Huei-Ru MJ, Sichao Ma, Paul JAK. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2013;2:191–199. doi: 10.1016/j.coche.2013.03.005. - DOI
-
- Hori Y, Murata A, Takahashi R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1989;85:2309–2326. doi: 10.1039/f19898502309. - DOI
-
- Akira M, Hori Y. Product selectivity affected by cationic species in electrochemical reduction of CO2 and CO at a Cu electrode. Bull. Chem. Soc. Jpn. 1991;64:123–127. doi: 10.1246/bcsj.64.123. - DOI
-
- Varela AS, Kroschel M, Reier T, Strasser P. Controlling the selectivity of CO2 electroreduction on copper: the effect of the electrolyte concentration and the importance of the local pH. Catal. Today. 2015;260:8–13. doi: 10.1016/j.cattod.2015.06.009. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

