Characterization and Antibiotic Sensitivity Profile of Bacteria in Orofacial Abscesses of Odontogenic Origin
- PMID: 29038627
- PMCID: PMC5628067
- DOI: 10.1007/s12663-016-0966-7
Characterization and Antibiotic Sensitivity Profile of Bacteria in Orofacial Abscesses of Odontogenic Origin
Abstract
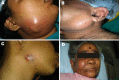
Background: Odontogenic infections range from peripheral abscess to superficial and deep infections leading to severe infections in head and neck region. This study was aimed to assess bacterial isolates responsible for orofacial infection of odontogenic origin and their drug susceptibility patterns so as to provide better perceptive for the management of odontogenic infections.
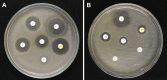
Methods: The study was made in a selected cohort of patients, irrespective of age and gender having moderate and severe orofacial infections of odontogenic origin admitted to Yenepoya University Hospital. Pus samples were collected and identification of bacteria was performed by 16S rRNA gene sequencing. Antimicrobial susceptibility testing was performed by Kirby-Bauer disc diffusion method.
Result: A total of 37 study subjects were included, with bacterial isolation rate of 31 (83.7 %). The mean age presented of all patients was 40.62. Of all, 24 (64.9 %) were males. Staphylococcus aureus, Enterobacter claocae subsp. dissolvens, Klebsiella quasipneumoniae subsp. similipneumoniae, Staphylococcus aureus subsp. anaerobius and Klebsiella pneumoniae subsp. ozaenae were the most prevalent isolates. Result showed that 58.6 % of the isolates were resistant to gentamicin, 52.5 % for ampicillin, 51.3 % for piperacillin; least resistant being 18.9 % for azithromycin.
Conclusion: High prevalence of bacterial isolates was found, Staphylococcus aureus being the dominant. Most of the bacteria were resistant to different classes of antibiotics. Appropriate antibiotics should be given based on the bacterial isolates, culture sensitivity and clinical course of the disease.
Keywords: 16S rRNA gene sequencing; Antibiotic susceptibility; Odontogenic infection; Orofacial abscesses.
Conflict of interest statement
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
