Clinical outcomes of residual or recurrent nasopharyngeal carcinoma treated with endoscopic nasopharyngectomy plus chemoradiotherapy or with chemoradiotherapy alone: a retrospective study
- PMID: 29038762
- PMCID: PMC5637710
- DOI: 10.7717/peerj.3912
Clinical outcomes of residual or recurrent nasopharyngeal carcinoma treated with endoscopic nasopharyngectomy plus chemoradiotherapy or with chemoradiotherapy alone: a retrospective study
Abstract
Background: Local residual and recurrent nasopharyngeal carcinoma (NPC) generally shows treatment failure after standard radiotherapy with or without concurrent chemotherapy. Whether endoscopic nasopharyngectomy might provide an additional therapeutic advantage remains controversial. Therefore, we retrospectively compared the clinical prognoses of patients with residual or recurrent NPC treated with endoscopic nasopharyngectomy combined with chemoradiotherapy (CRT) with those of patients treated with CRT alone.
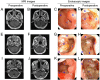
Methods and materials: A total of sixty-two patients with local residual or recurrent NPC were studied retrospectively: 36 patients received endoscopic nasopharyngectomy combined with CRT, whereas 26 patients who refused the surgery or had surgical contraindications received CRT alone. Serum Epstein-Barr virus (EBV) DNA levels were measured pre- and post-treatment. The differences in prognosis between the two treatment regimens and the pre- and post-treatment changes in EBV-DNA levels were analyzed.
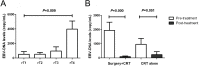
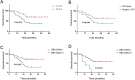
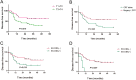
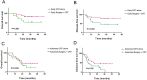
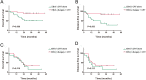
Results: The median follow-up time was 31 months, with a 3-year overall survival (OS) of 51.40% and a 3-year disease-free survival (DFS) of 46.86%. The surgery + CRT group had a better OS than the CRT alone group did (χ2 = 4.054, P = 0.044). The pretreatment EBV-DNA levels showed a positive correlation with the clinical staging of recurrent NPC (χ2 = 11.674, P = 0.009). Patients with negative pretreatment serum EBV-DNA levels showed a superior OS to those of patients who tested positive for EBV-DNA (>0 copy/mL) (χ2 = 9.833, P = 0.002). The post-treatment EBV-DNA levels, compared with the pretreatment levels, decreased significantly in the surgery + CRT group (Z = - 3.484, P = 0.000). In contrast, the EBV-DNA levels after CRT alone did not decrease significantly (Z = - 1.956, P = 0.051). Multivariate analysis indicated that local staging, pretreatment EBV-DNA load, and the treatment method were independent risk factors for OS. Subgroup analysis indicated that the patients who tested negative for EBV-DNA before the treatment and those who received surgery + CRT showed a better OS than those who received CRT alone.
Conclusions: The pretreatment serum EBV-DNA level was associated with disease prognosis. The combination therapy preceded by surgery can effectively decrease the copy number of EBV-DNA. Patients with local intermediate- and late-stage NPC, especially those negative for EBV-DNA, may consider opting for surgery followed by post-operative adjuvant radiotherapy or chemotherapy.
Keywords: Nasopharyngeal carcinoma; Prognosis; Recurrent tumor; Residual tumor; Serum epstein-barr virus DNA load.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
Similar articles
-
Integrating postradiotherapy plasma Epstein-Barr virus DNA and TNM stage for risk stratification of nasopharyngeal carcinoma to adjuvant therapy.Ann Oncol. 2020 Jun;31(6):769-779. doi: 10.1016/j.annonc.2020.03.289. Epub 2020 Mar 23. Ann Oncol. 2020. PMID: 32217076
-
Neoadjuvant or Adjuvant Chemotherapy Plus Concurrent CRT Versus Concurrent CRT Alone in the Treatment of Nasopharyngeal Carcinoma: A Study Based on EBV DNA.J Natl Compr Canc Netw. 2019 Jun 1;17(6):703-710. doi: 10.6004/jnccn.2018.7270. J Natl Compr Canc Netw. 2019. PMID: 31200353
-
Survival analysis of patients with advanced-stage nasopharyngeal carcinoma according to the Epstein-Barr virus status.Oncotarget. 2016 Apr 26;7(17):24208-16. doi: 10.18632/oncotarget.8144. Oncotarget. 2016. PMID: 27008701 Free PMC article.
-
The emerging data on choice of optimal therapy for locally advanced nasopharyngeal carcinoma.Curr Opin Oncol. 2020 May;32(3):187-195. doi: 10.1097/CCO.0000000000000622. Curr Opin Oncol. 2020. PMID: 32175925 Review.
-
How successful is high-dose (> or = 60 Gy) reirradiation using mainly external beams in salvaging local failures of nasopharyngeal carcinoma?Int J Radiat Oncol Biol Phys. 1998 Mar 1;40(4):897-913. doi: 10.1016/s0360-3016(97)00854-7. Int J Radiat Oncol Biol Phys. 1998. PMID: 9531376 Review.
Cited by
-
Prognostic value of Epstein-Barr virus DNA load in nasopharyngeal carcinoma: a meta-analysis.Pan Afr Med J. 2022 Jan 3;41:6. doi: 10.11604/pamj.2022.41.6.28946. eCollection 2022. Pan Afr Med J. 2022. PMID: 35145598 Free PMC article. Review.
-
Margins in oncologic nasopharyngeal surgery: a systematic review with meta-analysis.Acta Otorhinolaryngol Ital. 2025 May;45(Suppl. 1):S56-S70. doi: 10.14639/0392-100X-suppl.1-45-2025-N1170. Acta Otorhinolaryngol Ital. 2025. PMID: 40400377 Free PMC article.
-
Clinical characteristics and prognostic value of pre-retreatment plasma epstein-barr virus DNA in locoregional recurrent nasopharyngeal carcinoma.Cancer Med. 2019 Aug;8(10):4633-4643. doi: 10.1002/cam4.2339. Epub 2019 Jul 3. Cancer Med. 2019. PMID: 31268626 Free PMC article.
-
Enhancing Nasopharyngeal Carcinoma Survival Prediction: Integrating Pre- and Post-Treatment MRI Radiomics with Clinical Data.J Imaging Inform Med. 2024 Oct;37(5):2474-2489. doi: 10.1007/s10278-024-01109-7. Epub 2024 Apr 30. J Imaging Inform Med. 2024. PMID: 38689151 Free PMC article.
-
Comparing the Effectiveness of Endoscopic Surgeries With Intensity-Modulated Radiotherapy for Recurrent rT3 and rT4 Nasopharyngeal Carcinoma: A Meta-Analysis.Front Oncol. 2021 Jul 26;11:703954. doi: 10.3389/fonc.2021.703954. eCollection 2021. Front Oncol. 2021. PMID: 34381725 Free PMC article.
References
-
- Chan JY, Wei WI. Critical appraisal of maxillary swing approach for nasopharyngeal carcinoma. Expert Opinion on Therapeutic Targets. 2012;16(Suppl 1):S111–S117. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

