Malignant bone tumors (other than Ewing's): clinical practice guidelines for diagnosis, treatment and follow-up by Spanish Group for Research on Sarcomas (GEIS)
- PMID: 29038849
- PMCID: PMC5686259
- DOI: 10.1007/s00280-017-3436-0
Malignant bone tumors (other than Ewing's): clinical practice guidelines for diagnosis, treatment and follow-up by Spanish Group for Research on Sarcomas (GEIS)
Abstract
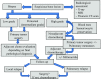
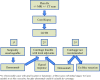
Primary malignant bone tumors are uncommon and heterogeneous malignancies. This document is a guideline developed by the Spanish Group for Research on Sarcoma with the participation of different specialists involved in the diagnosis and treatment of bone sarcomas. The aim is to provide practical recommendations with the intention of helping in the clinical decision-making process. The diagnosis and treatment of bone tumors requires a multidisciplinary approach, involving as a minimum pathologists, radiologists, surgeons, and radiation and medical oncologists. Early referral to a specialist center could improve patients' survival. The multidisciplinary management of osteosarcoma, chondrosarcoma, chordoma, giant cell tumor of bone and other rare bone tumors is reviewed in this guideline. Ewing's sarcoma will be the focus of a separate guideline because of its specific biological, clinical and therapeutic features. Each statement has been accompanied by the level of evidence and grade of recommendation on the basis of the available data. Surgical excision is the mainstay of treatment of a localized bone tumor, with various techniques available depending on the histologic type, grade and location of the tumor. Chemotherapy plays an important role in some chemosensitive subtypes (such as high-grade osteosarcoma). In other subtypes, historically considered chemoresistant (such as chordoma or giant cell tumor of bone), new targeted therapies have emerged recently, with a very significant efficacy in the case of denosumab. Radiation therapy is usually necessary in the treatment of chordoma and sometimes of other bone tumors.
Keywords: Bone sarcomas; Bone tumors; Clinical guideline; Diagnosis; Treatment.
Conflict of interest statement
Conflict of interest
The authors of this article S Bagué, R Correa, E Ortiz-Cruz, R Alvarez, J Martínez-Trufero, D Bernabeu, X García del Muro, J Cruz, A Santos and A López-Pousa, declare that they have no conflict of interest. The author A Redondo has been a part of an advisory board for Amgen and Novartis. The author C Valverde declares that she is a part of an advisory board for Denosumab drug. Besides, she is part of advisory boards for the next companies: Eisai, PharmaMar, Pfizer, Bayer and Lilly. The author of this article J Martín-Broto declares that he is a part of an advisory board for Novartis, Eisai and Lilly. Besides, he received Research Funding Grant from Novartis, PharmaMar and Eisai.
Studies involving in human participants
This article does not contain any new studies with human participants performed by any of the authors.
Figures
References
-
- Somerfield M, Padberg J, Pfister D, et al. ASCO clinical practice guidelines: process, progress, pitfalls, and prospects. Classic Pap Curr Comments. 2000;4:881–886.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical