Lymph node density as a prognostic predictor in patients with betel nut-related oral squamous cell carcinoma
- PMID: 29038963
- PMCID: PMC5866838
- DOI: 10.1007/s00784-017-2247-3
Lymph node density as a prognostic predictor in patients with betel nut-related oral squamous cell carcinoma
Abstract
Objectives: Lymph node metastasis in oral squamous cell carcinoma (OSCC) is a poor prognostic factor. The histopathologic stage (e.g., pN) is used to evaluate the severity of lymph node metastasis; however, the current staging system insufficiently predicts survival and recurrence. We investigated clinical outcomes and lymph node density (LND) in betel nut-chewing individuals.
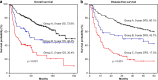
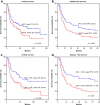
Material and methods: We retrospectively analyzed 389 betel nut-exposed patients with primary OSCC who underwent surgical resection in 2002-2015. The prognostic significance of LND was evaluated by overall survival (OS) and disease-free survival (DFS) using the Kaplan-Meier method.
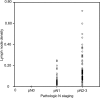
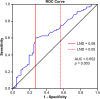
Results: Kaplan-Meier analyses showed that the 5-year OS and DFS rates in all patients were 60.9 and 48.9%, respectively. Multivariate analysis showed that variables independently prognostic for OS were aged population (hazard ratio [HR] = 1.6, 95% confidence interval [95% CI] = 1.1-2.5; P = .025), and cell differentiation classification (HR = 2.4, 95% CI = 1.4-4.2; P = .002). In pathologic N-positive patients, a receiver operating characteristic (ROC) curve for OS was used and indicated the best cutoff of 0.05, and the multivariate analysis showed that LND was an independent predictor of OS (HR = 2.2, 95% CI = 1.3-3.7; P = .004).
Conclusions: Lymph node density, at a cutoff of 0.05, was an independent predictor of OS and DFS. OS and DFS underwent multiple analyses, and LND remained significant. The pathologic N stage had no influence in the OS analysis.
Clinical relevance: LND is a more reliable predictor of survival in betel nut-chewing patients for further post operation adjuvant treatment, such as reoperation or adjuvant radiotherapy.
Keywords: Betel nut; Lymph node density; Oral squamous cell carcinoma; Prognostic factor.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All the patients provided informed consent and the Ethical Committee of the Tri-Service General Hospital (Taipei, Taiwan) approved the retrospective study (institutional review board protocol no: I-105-05-049).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures
Similar articles
-
Prognostic value of lymph node density in node-positive patients with oral squamous cell carcinoma.Ann Surg Oncol. 2011 Aug;18(8):2310-7. doi: 10.1245/s10434-011-1614-6. Epub 2011 Feb 20. Ann Surg Oncol. 2011. PMID: 21336511
-
Prognostic value of lymph node density in buccal squamous cell carcinoma.Am J Otolaryngol. 2017 Sep-Oct;38(5):529-532. doi: 10.1016/j.amjoto.2017.05.001. Epub 2017 May 20. Am J Otolaryngol. 2017. PMID: 28554580
-
Lymph node density is a significant predictor of outcome in patients with oral cancer.Cancer. 2009 Dec 15;115(24):5700-10. doi: 10.1002/cncr.24631. Cancer. 2009. PMID: 19691095
-
Lymph node ratio as prognostic variable in oral squamous cell carcinomas: Systematic review and meta-analysis.Oral Oncol. 2019 Feb;89:133-143. doi: 10.1016/j.oraloncology.2018.12.032. Epub 2019 Jan 8. Oral Oncol. 2019. PMID: 30732951
-
Prognostic value of the nodal yield in oral squamous cell carcinoma: a systematic review and meta-analysis.Expert Rev Anticancer Ther. 2023 Mar;23(3):339-345. doi: 10.1080/14737140.2023.2168648. Epub 2023 Jan 22. Expert Rev Anticancer Ther. 2023. PMID: 36645663
Cited by
-
Towards an Improved Pathological Node Classification for Prognostic Stratification of Patients With Oral Cavity Squamous Cell Carcinoma: Results From a Nationwide Registry Study.Front Oncol. 2022 Jun 28;12:910158. doi: 10.3389/fonc.2022.910158. eCollection 2022. Front Oncol. 2022. PMID: 35837108 Free PMC article.
-
Comparison of the hospitalization period after microvascular reconstruction flap in trismus patients: free anterolateral thigh flap versus free forearm flap.Clin Oral Investig. 2019 Jul;23(7):2951-2957. doi: 10.1007/s00784-018-02799-4. Epub 2019 Jan 7. Clin Oral Investig. 2019. PMID: 30617585
-
Molecular Interplays Between Cell Invasion and Radioresistance That Lead to Poor Prognosis in Head-Neck Cancer.Front Oncol. 2021 Jul 9;11:681717. doi: 10.3389/fonc.2021.681717. eCollection 2021. Front Oncol. 2021. PMID: 34307149 Free PMC article.
-
ECM Remodeling in Squamous Cell Carcinoma of the Aerodigestive Tract: Pathways for Cancer Dissemination and Emerging Biomarkers.Cancers (Basel). 2021 Jun 2;13(11):2759. doi: 10.3390/cancers13112759. Cancers (Basel). 2021. PMID: 34199373 Free PMC article. Review.
-
Prognostic value of lymph node density on cancer staging system for gastric cancer without distal metastasis: a population-based analysis of SEER database.World J Surg Oncol. 2022 Sep 29;20(1):325. doi: 10.1186/s12957-022-02795-9. World J Surg Oncol. 2022. PMID: 36175896 Free PMC article.
References
-
- Kao SY, Lim E. An overview of detection and screening of oral cancer in Taiwan. Chin J Dent Res. 2015;18:7–12. - PubMed
-
- Head and Neck Cancers (Version 2.2016). (Accessed October 16, 2016, at https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf)
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

