Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage
- PMID: 29039412
- PMCID: PMC5674160
- DOI: 10.1038/cr.2017.126
Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage
Abstract
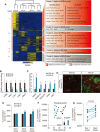
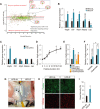
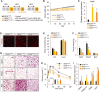
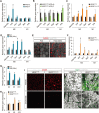
Intermittent fasting (IF), a periodic energy restriction, has been shown to provide health benefits equivalent to prolonged fasting or caloric restriction. However, our understanding of the underlying mechanisms of IF-mediated metabolic benefits is limited. Here we show that isocaloric IF improves metabolic homeostasis against diet-induced obesity and metabolic dysfunction primarily through adipose thermogenesis in mice. IF-induced metabolic benefits require fasting-mediated increases of vascular endothelial growth factor (VEGF) expression in white adipose tissue (WAT). Furthermore, periodic adipose-VEGF overexpression could recapitulate the metabolic improvement of IF in non-fasted animals. Importantly, fasting and adipose-VEGF induce alternative activation of adipose macrophage, which is critical for thermogenesis. Human adipose gene analysis further revealed a positive correlation of adipose VEGF-M2 macrophage-WAT browning axis. The present study uncovers the molecular mechanism of IF-mediated metabolic benefit and suggests that isocaloric IF can be a preventive and therapeutic approach against obesity and metabolic disorders.
Figures



Comment in
-
White adipose tissue coloring by intermittent fasting.Cell Res. 2017 Nov;27(11):1300-1301. doi: 10.1038/cr.2017.130. Epub 2017 Oct 24. Cell Res. 2017. PMID: 29097740 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

