MicroRNA‑218 promotes prostaglandin E2 to inhibit osteogenic differentiation in synovial mesenchymal stem cells by targeting 15‑hydroxyprostaglandin dehydrogenase [NAD(+)]
- PMID: 29039590
- PMCID: PMC5779987
- DOI: 10.3892/mmr.2017.7795
MicroRNA‑218 promotes prostaglandin E2 to inhibit osteogenic differentiation in synovial mesenchymal stem cells by targeting 15‑hydroxyprostaglandin dehydrogenase [NAD(+)]
Abstract
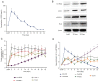
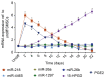
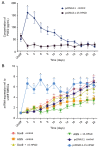
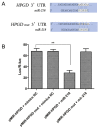
The chondrogenic differentiation of synovial mesenchymal stem cells (SMSCs) is regulated by essential transcription factors and signaling cascades. However, the precise mechanisms involved in this process remain unclear. MicroRNAs (miRs/miRNAs) are undersized non‑coding RNAs responsible for the post‑transcriptional regulation of gene expression, by binding to the 3'‑untranslated regions (3'‑UTRs) of their target mRNAs. miRNAs may constitute a promising tool to regulate SMSC differentiation and to advance the controlled differentiation of SMSCs in therapeutic applications. The aim of the present study was to examine the role of miR‑218 in SMSC differentiation towards chondrocytes. The present study comparatively analyzed the expression profile of known miRNAs and specific target genes in SMSCs between early and late differentiation stages. Western blotting and reverse transcription‑quantitative polymerase chain reaction analysis of gene expression demonstrated the upregulation of 15‑hydroxyprostaglandin dehydrogenase [NAD(+)] (15‑HPGD), prostaglandin E2 (PGE2) and rate limiting enzymes responsible for the synthesis of PGE2 precursors throughout chondrogenesis. Through correlation analysis, it was observed that there was a significant association between miR‑128, 15‑HPGD gene expression, 15‑HPGD protein expression and microsomal prostaglandin E synthase 1. Further experiments demonstrated that miR‑218 decreased PGE2 concentration by binding to the 3'‑UTR of 15‑HPGD. Using an immunofluorescence reporting system, it was observed that miR‑218 regulated the expression of 15‑HPGD during the differentiation of SMSCs into cartilage, and subsequently inhibited osteogenesis during chondrogenesis by acting on the 3'UTR of 15‑HPGD. Therefore, miR‑218 may be an important regulator targeting osteogenic factors and modulating cartilage formation and differentiation. The results of the present study provided a novel insight beneficial to cellular manipulation methods during cartilage regeneration, and in cartilage tissue engineering research.
Figures
References
-
- Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843–853. doi: 10.1002/art.21651. - DOI - PubMed
-
- Alm JJ, Heino TJ, Hentunen TA, Väänänen HK, Aro HT. Transient 100 nM dexamethasone treatment reduces inter- and intraindividual variations in osteoblastic differentiation of bone marrow-derived human mesenchymal stem cells. Tissue Eng Part C Methods. 2012;18:658–666. doi: 10.1089/ten.tec.2011.0675. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

