Single-Dose Pharmacokinetics of HLD200, a Delayed-Release and Extended-Release Methylphenidate Formulation, in Healthy Adults and in Adolescents and Children with Attention-Deficit/Hyperactivity Disorder
- PMID: 29039979
- PMCID: PMC5771548
- DOI: 10.1089/cap.2017.0044
Single-Dose Pharmacokinetics of HLD200, a Delayed-Release and Extended-Release Methylphenidate Formulation, in Healthy Adults and in Adolescents and Children with Attention-Deficit/Hyperactivity Disorder
Abstract
Objective: Current extended-release (ER) formulations of psychostimulants used for treatment of attention-deficit/hyperactivity disorder (ADHD) provide an extended duration of ADHD symptom control; however, the onset of efficacy can be protracted and variable, leaving the early morning untreated. The primary objective was to characterize the single-dose pharmacokinetics and tolerability of HLD200, an evening-dosed, delayed-release (DR) and ER formulation of methylphenidate (MPH), in healthy adults and in adolescents and children with ADHD.
Methods: The pharmacokinetics and tolerability of a single, oral evening dose of HLD200 (54 mg) were evaluated in two single-center open-label studies: the first in healthy adults (n = 12) and the second in adolescents (n = 18) and children (n = 11) with ADHD. Primary pharmacokinetic endpoints were the rate and extent of MPH absorption (Cmax and area under the curve [AUC]) and time to peak concentration (Tmax). These parameters were calculated using noncompartmental analysis.
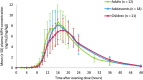
Results: HLD200 produced a pharmacokinetic profile characterized by an 8- to 10-hour delay in MPH release, followed by a period of extended controlled release, resulting in an ascending absorption profile that coincided with the early morning and afternoon. Mean values (coefficient of variation [CV]%) of weight-adjusted pharmacokinetic parameters were similar in adults and in adolescents and children with ADHD: Cmax ([ng/mL]/[mg/kg]) was 9.1 (35.2), 8.8 (34.5), and 7.4 (30.1); AUC0-t ([ng · h/mL]/[mg/kg]) was 126.5 (35.5), 129.4 (34.8), and 129.7 (27.3); and Tmax (hours) was 15.6 (11.1), 17.1 (14.5), and 17.7 (14.1), respectively. Intersubject variability in the mean time to achieve ascending plasma MPH concentrations of 2, 3, 4, and 5 ng/mL was low (CV: 7.8%-17.7%).
Conclusions: Evening-dosed HLD200 produces the intended DR and ER pharmacokinetic profile that provides a consistent predictable delay in initial MPH release until the early morning, followed by extended release across the day. The body weight-adjusted pharmacokinetics of HLD200 were similar between adults and adolescents and children with ADHD.
Trial registration: ClinicalTrials.gov NCT01907360.
Keywords: attention-deficit/hyperactivity disorder; delayed-release; extended-release; methylphenidate; pharmacokinetics.
Figures
References
-
- Bai JP, Burckart GJ, Mulberg AE: Literature review of gastrointestinal physiology in the elderly, in pediatric patients, and in patients with gastrointestinal diseases. J Pharm Sci 105:476–483, 2016 - PubMed
-
- Birmaher B, Greenhill LL, Cooper TB, Fried J, Maminski B. Sustained release methylphenidate: Pharmacokinetic studies in ADDH males. J Am Acad Child Adolesc Psychiatry 28:768–772, 1989 - PubMed
-
- Childress A, Tran C: Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs 25:463–474, 2016 - PubMed
-
- Childress AC: Methylphenidate HCL for the treatment of ADHD in children and adolescents. Expert Opin Pharmacother 17:1171–1178, 2016 - PubMed
-
- Faraone SV, Schachar RJ, Barkley RA, Nullmeier R, Sallee FR: Early morning functional impairments in stimulant-treated children with attention-deficit/hyperactivity disorder versus controls: Impact on the family. J Child Adolesc Psychopharmacol [Epub ahead of print]; DOI: 10.1089/cap.2016.0164, 2017 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

