Nivolumab Plus Ipilimumab in Patients With Advanced Melanoma: Updated Survival, Response, and Safety Data in a Phase I Dose-Escalation Study
- PMID: 29040030
- PMCID: PMC5946731
- DOI: 10.1200/JCO.2017.72.2850
Nivolumab Plus Ipilimumab in Patients With Advanced Melanoma: Updated Survival, Response, and Safety Data in a Phase I Dose-Escalation Study
Abstract
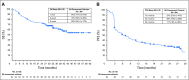
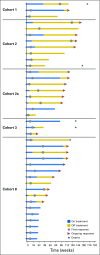
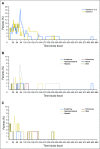
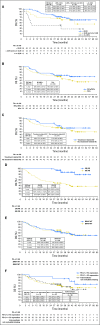
Purpose The clinical activity observed in a phase I dose-escalation study of concurrent therapy with nivolumab (NIVO) and ipilimumab (IPI) in patients with previously treated or untreated advanced melanoma led to subsequent clinical development, including randomized trials. Here, we report long-term follow-up data from study CA209-004, including 3-year overall survival (OS). Patients and Methods Concurrent cohorts 1, 2, 2a, and 3 received escalating doses of NIVO plus IPI once every 3 weeks for four doses, followed by NIVO once every 3 weeks for four doses, then NIVO plus IPI once every 12 weeks for eight doses. An expansion cohort (cohort 8) received concurrent NIVO 1 mg/kg plus IPI 3 mg/kg once every 3 weeks for four doses, followed by NIVO 3 mg/kg once every 2 weeks, which is the dose and schedule used in phase II and III studies and now approved for patients with unresectable or metastatic melanoma. Results Among all concurrent cohorts (N = 94) at a follow-up of 30.3 to 55.0 months, the 3-year OS rate was 63% and median OS had not been reached. Objective response rate by modified WHO criteria was 42%, and median duration of response was 22.3 months. Incidence of grade 3 and 4 treatment-related adverse events was 59%. The most common grade 3 and 4 treatment-related adverse events were increases in lipase (15%), alanine aminotransferase (12%), and aspartate aminotransferase (11%). One treatment-related death (1.1%) occurred in a patient who had multiorgan failure 70 days after the last dose of NIVO plus IPI. Conclusion This is the longest follow-up for NIVO plus IPI combination therapy in patients with advanced melanoma. The 3-year OS rate of 63% is the highest observed for this patient population and provides additional evidence for the durable clinical activity of immune checkpoint inhibitors in the treatment of advanced melanoma.
Trial registration: ClinicalTrials.gov NCT01024231.
Figures

Comment in
-
The age of enlightenment in melanoma immunotherapy.J Immunother Cancer. 2018 Aug 22;6(1):80. doi: 10.1186/s40425-018-0397-8. J Immunother Cancer. 2018. PMID: 30134977 Free PMC article.
References
-
- Robert C, Schachter J, Long GV, et al. : Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521-2532, 2015 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

