Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation
- PMID: 29040031
- PMCID: PMC8462523
- DOI: 10.1200/JCO.2017.75.8177
Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation
Abstract
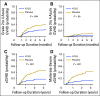
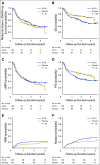
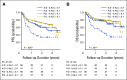
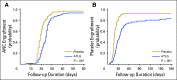
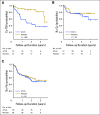
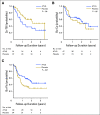
Purpose Several open-label randomized studies have suggested that in vivo T-cell depletion with anti-T-lymphocyte globulin (ATLG; formerly antithymocyte globulin-Fresenius) reduces chronic graft-versus-host disease (cGVHD) without compromising survival. We report a prospective, double-blind phase III trial to investigate the effect of ATLG (Neovii Biotech, Lexington, MA) on cGVHD-free survival. Patients and Methods Two hundred fifty-four patients 18 to 65 years of age with acute leukemia or myelodysplastic syndrome who underwent myeloablative HLA-matched unrelated hematopoietic cell transplantation (HCT) were randomly assigned one to one to placebo (n =128 placebo) or ATLG (n = 126) treatment at 27 sites. Patients received either ATLG or placebo 20 mg/kg per day on days -3, -2, -1 in addition to tacrolimus and methotrexate as GVHD prophylaxis. The primary study end point was moderate-severe cGVHD-free survival. Results Despite a reduction in grade 2 to 4 acute GVHD (23% v 40%; P = .004) and moderate-severe cGVHD (12% v 33%; P < .001) in ATLG recipients, no difference in moderate-severe cGVHD-free survival between ATLG and placebo was found (2-year estimate: 48% v 44%, respectively; P = .47). Both progression-free survival (PFS) and overall survival (OS) were lower with ATLG (2-year estimate: 47% v 65% [ P = .04] and 59% v 74% [ P = .034], respectively). Multivariable analysis confirmed that ATLG was associated with inferior PFS (hazard ratio, 1.55; 95% CI, 1.05 to 2.28; P = .026) and OS (hazard ratio, 1.74; 95% CI, 1.12 to 2.71; P = .01). Conclusion In this prospective, randomized, double-blind trial of ATLG in unrelated myeloablative HCT, the incorporation of ATLG did not improve moderate-severe cGVHD-free survival. Moderate-severe cGVHD was significantly lower with ATLG, but PFS and OS also were lower. Additional analyses are needed to understand the appropriate role for ATLG in HCT.
Conflict of interest statement
Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti–T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease–Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation
Robert J. Soiffer
Haesook T. Kim
Joseph McGuirk
Mitchell E. Horwitz
No relationship to disclose
Laura Johnston
No relationship to disclose
Mrinal M. Patnaik
No relationship to disclose
Witold Rybka
Andrew Artz
David L. Porter
No relationship to disclose
Thomas C. Shea
Michael W. Boyer
Richard T. Maziarz
Paul J. Shaughnessy
Usama Gergis
Hana Safah
Ran Reshef
John F. DiPersio
Patrick J. Stiff
No relationship to disclose
Madhuri Vusirikala
Jeff Szer
Jennifer Holter
No relationship to disclose
James D. Levine
Paul J. Martin
Joseph A. Pidala
No relationship to disclose
Ian D. Lewis
Vincent T. Ho
Edwin P. Alyea
No relationship to disclose
Jerome Ritz
Frank Glavin
Peter Westervelt
No relationship to disclose
Madan H. Jagasia
No relationship to disclose
Yi-Bin Chen
Figures


Comment in
-
Antithymocyte Globulin for Graft-Versus-Host Disease Prophylaxis After Allogeneic Hematopoietic Stem-Cell Transplantation.J Clin Oncol. 2017 Dec 20;35(36):3993-3995. doi: 10.1200/JCO.2017.76.0512. Epub 2017 Oct 31. J Clin Oncol. 2017. PMID: 29087771 No abstract available.
-
Reply to J.J. Boelens et al.J Clin Oncol. 2018 Apr 10;36(11):1177-1178. doi: 10.1200/JCO.2017.77.2947. Epub 2018 Feb 7. J Clin Oncol. 2018. PMID: 29412782 No abstract available.
-
Reply to J.J. Boelens et al.J Clin Oncol. 2018 Apr 10;36(11):1176-1177. doi: 10.1200/JCO.2017.77.2939. Epub 2018 Feb 7. J Clin Oncol. 2018. PMID: 29412783 No abstract available.
-
Fine-Tuning Antithymocyte Globulin Dosing and Harmonizing Clinical Trial Design.J Clin Oncol. 2018 Apr 10;36(11):1175-1176. doi: 10.1200/JCO.2017.77.1774. Epub 2018 Feb 7. J Clin Oncol. 2018. PMID: 29412785 No abstract available.
Similar articles
-
Acute GVHD prophylaxis plus ATLG after myeloablative allogeneic haemopoietic peripheral blood stem-cell transplantation from HLA-identical siblings in patients with acute myeloid leukaemia in remission: final results of quality of life and long-term outcome analysis of a phase 3 randomised study.Lancet Haematol. 2019 Feb;6(2):e89-e99. doi: 10.1016/S2352-3026(18)30214-X. Lancet Haematol. 2019. PMID: 30709437 Clinical Trial.
-
Effect of Antihuman T Lymphocyte Globulin on Immune Recovery after Myeloablative Allogeneic Stem Cell Transplantation with Matched Unrelated Donors: Analysis of Immune Reconstitution in a Double-Blind Randomized Controlled Trial.Biol Blood Marrow Transplant. 2018 Nov;24(11):2216-2223. doi: 10.1016/j.bbmt.2018.07.002. Epub 2018 Jul 10. Biol Blood Marrow Transplant. 2018. PMID: 30006305 Clinical Trial.
-
Efficacy of two different doses of rabbit anti-T-lymphocyte globulin to prevent graft-versus-host disease in children with haematological malignancies transplanted from an unrelated donor: a multicentre, randomised, open-label, phase 3 trial.Lancet Oncol. 2017 Aug;18(8):1126-1136. doi: 10.1016/S1470-2045(17)30417-5. Epub 2017 Jul 10. Lancet Oncol. 2017. PMID: 28705454 Clinical Trial.
-
Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial.Lancet Haematol. 2017 Jun;4(6):e293-e301. doi: 10.1016/S2352-3026(17)30081-9. Lancet Haematol. 2017. PMID: 28583289 Clinical Trial.
-
Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel.Bone Marrow Transplant. 2020 Jun;55(6):1093-1102. doi: 10.1038/s41409-020-0792-x. Epub 2020 Jan 22. Bone Marrow Transplant. 2020. PMID: 31969678 Free PMC article. Review.
Cited by
-
Graft-versus-host disease and impact on relapse in myelofibrosis undergoing hematopoietic stem cell transplantation.Bone Marrow Transplant. 2024 Apr;59(4):550-557. doi: 10.1038/s41409-024-02220-7. Epub 2024 Feb 6. Bone Marrow Transplant. 2024. PMID: 38321269 Free PMC article.
-
Controversies and expectations for the prevention of GVHD: A biological and clinical perspective.Front Immunol. 2022 Nov 23;13:1057694. doi: 10.3389/fimmu.2022.1057694. eCollection 2022. Front Immunol. 2022. PMID: 36505500 Free PMC article. Review.
-
The graft versus leukemia effect: donor lymphocyte infusions and cellular therapy.Front Immunol. 2024 Mar 15;15:1328858. doi: 10.3389/fimmu.2024.1328858. eCollection 2024. Front Immunol. 2024. PMID: 38558819 Free PMC article. Review.
-
Comparison of myeloablative and reduced intensity conditioning unrelated donor allogeneic peripheral blood stem cell transplant outcomes for AML using thymoglobulin for GVHD prophylaxis.Ann Hematol. 2021 Apr;100(4):969-978. doi: 10.1007/s00277-021-04445-8. Epub 2021 Feb 16. Ann Hematol. 2021. PMID: 33594448 Free PMC article.
-
Allogeneic hematopoietic cell transplantation after prior targeted therapy for high-risk chronic lymphocytic leukemia.Blood Adv. 2020 Sep 8;4(17):4113-4123. doi: 10.1182/bloodadvances.2020002184. Blood Adv. 2020. PMID: 32882002 Free PMC article.
References
-
- Lee SJ, Kim HT, Ho VT, et al. : Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant 38:305-310, 2006 - PubMed
-
- Bacigalupo A, Lamparelli T, Barisione G, et al. : Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: Long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant 12:560-565, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

