Failure of Isoflurane Cardiac Preconditioning in Obese Type 2 Diabetic Mice Involves Aberrant Regulation of MicroRNA-21, Endothelial Nitric-oxide Synthase, and Mitochondrial Complex I
- PMID: 29040168
- PMCID: PMC5726897
- DOI: 10.1097/ALN.0000000000001926
Failure of Isoflurane Cardiac Preconditioning in Obese Type 2 Diabetic Mice Involves Aberrant Regulation of MicroRNA-21, Endothelial Nitric-oxide Synthase, and Mitochondrial Complex I
Abstract
Background: Diabetes impairs the cardioprotective effect of volatile anesthetics, yet the mechanisms are still murky. We examined the regulatory effect of isoflurane on microRNA-21, endothelial nitric-oxide synthase, and mitochondrial respiratory complex I in type 2 diabetic mice.
Methods: Myocardial ischemia/reperfusion injury was produced in obese type 2 diabetic (db/db) and C57BL/6 control mice ex vivo in the presence or absence of isoflurane administered before ischemia. Cardiac microRNA-21 was quantified by real-time quantitative reverse transcriptional-polymerase chain reaction. The dimers and monomers of endothelial nitric-oxide synthase were measured by Western blot analysis. Mitochondrial nicotinamide adenine dinucleotide fluorescence was determined in Langendorff-perfused hearts.
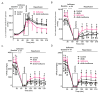
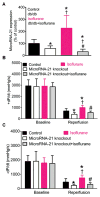
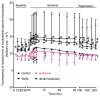
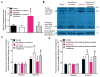
Results: Body weight and fasting blood glucose were greater in db/db than C57BL/6 mice. Isoflurane decreased left ventricular end-diastolic pressure from 35 ± 8 mmHg in control to 23 ± 9 mmHg (P = 0.019, n = 8 mice/group, mean ± SD) and elevated ±dP/dt 2 h after post-ischemic reperfusion in C57BL/6 mice. These beneficial effects of isoflurane were lost in db/db mice. Isoflurane elevated microRNA-21 and the ratio of endothelial nitric-oxide synthase dimers/monomers and decreased mitochondrial nicotinamide adenine dinucleotide levels 5 min after ischemia in C57BL/6 but not db/db mice. MicroRNA-21 knockout blocked these favorable effects of isoflurane, whereas endothelial nitric-oxide synthase knockout had no effect on the expression of microRNA-21 but blocked the inhibitory effect of isoflurane preconditioning on nicotinamide adenine dinucleotide.
Conclusions: Failure of isoflurane cardiac preconditioning in obese type 2 diabetic db/db mice is associated with aberrant regulation of microRNA-21, endothelial nitric-oxide synthase, and mitochondrial respiratory complex I.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
MicroRNA-21 Mediates Isoflurane-induced Cardioprotection against Ischemia-Reperfusion Injury via Akt/Nitric Oxide Synthase/Mitochondrial Permeability Transition Pore Pathway.Anesthesiology. 2015 Oct;123(4):786-798. doi: 10.1097/ALN.0000000000000807. Anesthesiology. 2015. PMID: 26259139 Free PMC article.
-
Chronic Co-Administration of Sepiapterin and L-Citrulline Ameliorates Diabetic Cardiomyopathy and Myocardial Ischemia/Reperfusion Injury in Obese Type 2 Diabetic Mice.Circ Heart Fail. 2016 Jan;9(1):e002424. doi: 10.1161/CIRCHEARTFAILURE.115.002424. Circ Heart Fail. 2016. PMID: 26763290 Free PMC article.
-
Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism.Anesthesiology. 2010 Jan;112(1):73-85. doi: 10.1097/ALN.0b013e3181c4a607. Anesthesiology. 2010. PMID: 19996950 Free PMC article.
-
Endothelial nitric oxide synthase.Contrib Nephrol. 2011;170:93-101. doi: 10.1159/000324954. Epub 2011 Jun 9. Contrib Nephrol. 2011. PMID: 21659762 Review.
-
Role of microRNAs in cardiac preconditioning.J Cardiovasc Pharmacol. 2010 Dec;56(6):581-8. doi: 10.1097/FJC.0b013e3181f581ba. J Cardiovasc Pharmacol. 2010. PMID: 20980922 Free PMC article. Review.
Cited by
-
Guidelines on models of diabetic heart disease.Am J Physiol Heart Circ Physiol. 2022 Jul 1;323(1):H176-H200. doi: 10.1152/ajpheart.00058.2022. Epub 2022 Jun 3. Am J Physiol Heart Circ Physiol. 2022. PMID: 35657616 Free PMC article. Review.
-
Diabetes induces hepatocyte pyroptosis by promoting oxidative stress-mediated NLRP3 inflammasome activation during liver ischaemia and reperfusion injury.Ann Transl Med. 2020 Jun;8(12):739. doi: 10.21037/atm-20-1839. Ann Transl Med. 2020. PMID: 32647664 Free PMC article.
-
Cardio-omentopexy requires a cardioprotective innate immune response to promote myocardial angiogenesis in mice.JTCVS Open. 2022 Feb 24;10:222-242. doi: 10.1016/j.xjon.2022.02.027. eCollection 2022 Jun. JTCVS Open. 2022. PMID: 36004249 Free PMC article.
-
Current status and strategies of long noncoding RNA research for diabetic cardiomyopathy.BMC Cardiovasc Disord. 2018 Oct 20;18(1):197. doi: 10.1186/s12872-018-0939-5. BMC Cardiovasc Disord. 2018. PMID: 30342478 Free PMC article. Review.
-
Genetic deletion or pharmacologic inhibition of histone deacetylase 6 protects the heart against ischaemia/reperfusion injury by limiting tumour necrosis factor alpha-induced mitochondrial injury in experimental diabetes.Cardiovasc Res. 2024 Oct 14;120(12):1456-1471. doi: 10.1093/cvr/cvae144. Cardiovasc Res. 2024. PMID: 39001869 Free PMC article.
References
-
- Benedetto U, Caputo M, Vohra H, Davies A, Hillier J, Bryan A, Angelini GD. Off-pump versus on-pump coronary artery bypass surgery in patients with actively treated diabetes and multivessel coronary disease. J Thorac Cardiovasc Surg. 2016;152:1321–30. - PubMed
-
- Holzmann MJ, Rathsman B, Eliasson B, Kuhl J, Svensson AM, Nystrom T, Sartipy U. Long-term prognosis in patients with type 1 and 2 diabetes mellitus after coronary artery bypass grafting. J Am Coll Cardiol. 2015;65:1644–52. - PubMed
-
- Lee MC, Chen CH, Kuo MC, Kang PL, Lo A, Liu K. Isoflurane preconditioning-induced cardio-protection in patients undergoing coronary artery bypass grafting. Eur J Anaesthesiol. 2006;23:841–7. - PubMed
-
- Freiermuth D, Mets B, Bolliger D, Reuthebuch O, Doebele T, Scholz M, Gregor M, Haschke M, Seeberger MD, Fassl J. Sevoflurane and isoflurane-pharmacokinetics, hemodynamic stability, and cardioprotective effects during cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2016;30:1494–1501. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

