Analytical Pitfalls of Therapeutic Drug Monitoring of Thiopurines in Patients With Inflammatory Bowel Disease
- PMID: 29040228
- PMCID: PMC5690305
- DOI: 10.1097/FTD.0000000000000455
Analytical Pitfalls of Therapeutic Drug Monitoring of Thiopurines in Patients With Inflammatory Bowel Disease
Abstract
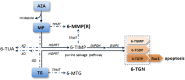
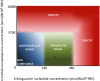
The use of thiopurines in the treatment of inflammatory bowel disease (IBD) can be optimized by the application of therapeutic drug monitoring. In this procedure, 6-thioguanine nucleotides (6-TGN) and 6-methylmercaptopurine (6-MMP) metabolites are monitored and related to therapeutic response and adverse events, respectively. Therapeutic drug monitoring of thiopurines, however, is hampered by several analytical limitations resulting in an impaired translation of metabolite levels to clinical outcome in IBD. Thiopurine metabolism is cell specific and requires nucleated cells and particular enzymes for 6-TGN formation. In the current therapeutic drug monitoring, metabolite levels are assessed in erythrocytes, whereas leukocytes are considered the main target cells of these drugs. Furthermore, currently used methods do not distinguish between active nucleotides and their unwanted residual products. Last, there is a lack of a standardized laboratorial procedure for metabolite assessment regarding the substantial instability of erythrocyte 6-TGN. To improve thiopurine therapy in patients with IBD, it is necessary to understand these limitations and recognize the general misconceptions in this procedure.
Conflict of interest statement
C. J. J. Mulder has served as a consultant and principal investigator for Teva Pharma B.V. A. A. van Bodegraven has served as a consultant or speaker for AbbVie, Ferring, Janssen, MSD, Pfizer, Takeda, TEVA, Tramedico, Vifor, and the Dutch Ministry of Health (ZonMW). He has received (unrestricted) research grants from Aventis and Ferring, and the Dutch Ministry of Health. N. K. H. de Boer has served as a speaker for AbbVie, Takeda, and MSD. He has served as a consultant and principal investigator for Takeda and Teva Pharma B.V. He has received a (unrestricted) research grant from Dr. Falk and Takeda. The remaining authors declare no conflict of interest.
Figures
References
-
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41–47. - PubMed
-
- Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008;8:24–36. - PubMed
-
- Derijks LJ, Gilissen LP, Hooymans PM, et al. Review article: thiopurines in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:715–729. - PubMed
-
- Zweiman B. Immunosuppressive effects of specific classes of agents with special reference to organ transplantation. Immunosuppression by thiopurines. Transpl Proc. 1973;5:1197–1201. - PubMed
-
- Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

