Preliminary 3-Year Evaluation of Experience With SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases
- PMID: 29040364
- PMCID: PMC5952962
- DOI: 10.1093/asj/sjx150
Preliminary 3-Year Evaluation of Experience With SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases
Erratum in
-
Corrigendum to: Preliminary 3-Year Evaluation of Experience With SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases.Aesthet Surg J. 2021 Apr 12;41(5):639. doi: 10.1093/asj/sjz124. Aesthet Surg J. 2021. PMID: 31067296 Free PMC article. No abstract available.
Abstract
Background: Silicone breast implants have been in use for breast augmentation for more than 50 years, but technological innovation has been lacking in implant design until recently.
Objectives: This study was designed to evaluate the complication and reoperation rates following breast augmentation utilizing the Motiva silicone breast implants.
Methods: This retrospective study evaluated the safety of Motiva implants in 5813 consecutive cases of breast augmentation. Implants with two different textured surfaces were evaluated: SilkSurface (nanotextured) and VelvetSurface (micro-textured).
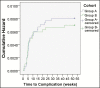
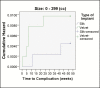
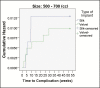
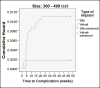
Results: Implants were placed between April 2013 and April 2016. A total of 44 complications were reported, with an overall complication rate of 0.76%, and the rate of reoperation was 0.76% over an interval of 3 years. There were no late complications and no cases of primary capsular contracture. No differences in complication rates were observed because of the implant date. However, among patients who received implants 300 to 499 cc in volume, complication rates were significantly lower with SilkSurface compared with VelvetSurface implants. Advanced statistical analysis supported the validity of the low complication rate reported in this study.
Conclusions: Overall, these findings suggest that Motiva silicone breast implants are associated with very low rates of complication and reoperation, and that the nano-textured SilkSurface implant is associated with fewer complications than micro-textured implants.
Figures




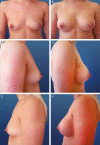
Comment in
-
Comments on "Preliminary 3-Year Evaluation of Experience With SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases".Aesthet Surg J. 2019 Jan 17;39(2):NP18-NP19. doi: 10.1093/asj/sjy203. Aesthet Surg J. 2019. PMID: 30517590 No abstract available.
-
Response to "Comments on 'Preliminary 3-Year Evaluation of Experience With SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases'".Aesthet Surg J. 2019 Jan 17;39(2):NP20-NP22. doi: 10.1093/asj/sjy272. Aesthet Surg J. 2019. PMID: 30517604 No abstract available.
-
Nano-Surface Implants: Indications and Limitations.Aesthet Surg J. 2021 Jul 14;41(8):NP1141-NP1142. doi: 10.1093/asj/sjaa265. Aesthet Surg J. 2021. PMID: 33128363 Free PMC article. No abstract available.
-
Response to: Nano-Surface Implants: Indications and Limitations.Aesthet Surg J. 2021 Jul 14;41(8):NP1143-NP1146. doi: 10.1093/asj/sjaa297. Aesthet Surg J. 2021. PMID: 33693485 Free PMC article. No abstract available.
References
-
- Bhushan B, Jung Y. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces. J Phys Condens Matter. 2008;20(22):225010.
-
- Valencia-Lazcano AA, Alonso-Rasgado T, Bayat A. Characterisation of breast implant surfaces and correlation with fibroblast adhesion. J Mech Behav Biomed Mater. 2013;21:133-148. - PubMed
-
- Sientra FDA Summary of Safety and Effectiveness Data (SSED) report http://www.accessdata.fda.gov/cdrh_docs/pdf7/p070004b.pdf. Accessed October 12, 2016.
-
- Cunningham B. The Mentor Core Study on silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120(7 Suppl 1):19S-29S; discussion 30S-32S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

