Outer inflammatory protein a (OipA) of Helicobacter pylori is regulated by host cell contact and mediates CagA translocation and interleukin-8 response only in the presence of a functional cag pathogenicity island type IV secretion system
- PMID: 29040466
- PMCID: PMC6433299
- DOI: 10.1093/femspd/ftx113
Outer inflammatory protein a (OipA) of Helicobacter pylori is regulated by host cell contact and mediates CagA translocation and interleukin-8 response only in the presence of a functional cag pathogenicity island type IV secretion system
Abstract
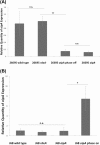
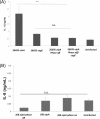
OipA is a phase-variable virulence factor of Helicobacter pylori. Mutations in oipA to turn the gene phase on in a cag pathogenicity island (PAI)-negative strain of H. pylori (J68) or phase off in a cag PAI-positive strain (26695) demonstrated that phase on oipA alleles in both strains had both increased oipA mRNA and human gastric adenocarcinoma (AGS) cell adherence compared to isogenic oipA phase off mutants. An oipA phase off mutant of H. pylori 26695 demonstrated decreased IL-8 secretion by AGS cells and failure to translocate the cag PAI effector CagA. Increased attachment by OipA expressing cag PAI-negative H. pylori J68 failed to alter secreted IL-8 levels. Thus, OipA is necessary but not sufficient for the induction of IL-8; however, it is necessary for translocation of the oncoprotein CagA. Perhaps the nearly invariant phase on status of oipA alleles among cag PAI-positive H. pylori isolates relates to the role of this outer membrane protein in effective translocation of CagA. oipA mRNA comparisons between AGS cell-adherent and non-adherent H. pylori 26695 revealed significantly greater levels in the adherent cells. This may allow H. pylori to adapt to conditions of host cell contact by altering expression of this virulence factor.
Keywords: CagA translocation; adhesin; interleukin-8; phase variation.
© FEMS 2017. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





Similar articles
-
Influence of oipA Phase Variation on Virulence Phenotypes Related to Type IV Secretion System in Helicobacter pylori.Helicobacter. 2024 Sep-Oct;29(5):e13140. doi: 10.1111/hel.13140. Helicobacter. 2024. PMID: 39440915
-
Helicobacter pylori CagA and Cag type IV secretion system activity have key roles in triggering gastric transcriptional and proteomic alterations.Infect Immun. 2025 Apr 8;93(4):e0059524. doi: 10.1128/iai.00595-24. Epub 2025 Mar 6. Infect Immun. 2025. PMID: 40047510 Free PMC article.
-
Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection.Gastroenterology. 2004 Apr;126(4):1030-43. doi: 10.1053/j.gastro.2003.12.048. Gastroenterology. 2004. PMID: 15057743
-
[The type IV secretion system encoded by the cag PAI of Helicobacter pylori].Wei Sheng Wu Xue Bao. 2007 Aug;47(4):743-5. Wei Sheng Wu Xue Bao. 2007. PMID: 17944386 Review. Chinese.
-
The Helicobacter pylori Cag Type IV Secretion System.Trends Microbiol. 2020 Aug;28(8):682-695. doi: 10.1016/j.tim.2020.02.004. Epub 2020 Mar 26. Trends Microbiol. 2020. PMID: 32451226 Free PMC article. Review.
Cited by
-
Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity.Toxins (Basel). 2019 Nov 19;11(11):677. doi: 10.3390/toxins11110677. Toxins (Basel). 2019. PMID: 31752394 Free PMC article. Review.
-
Identification of Pathogenicity Island Genes Associated with Loss of Type IV Secretion Function during Murine Infection with Helicobacter pylori.Infect Immun. 2020 May 20;88(6):e00801-19. doi: 10.1128/IAI.00801-19. Print 2020 May 20. Infect Immun. 2020. PMID: 32205402 Free PMC article.
-
Identification of interaction partners of outer inflammatory protein A: Computational and experimental insights into how Helicobacter pylori infects host cells.PLoS One. 2024 Oct 29;19(10):e0300557. doi: 10.1371/journal.pone.0300557. eCollection 2024. PLoS One. 2024. PMID: 39471168 Free PMC article.
-
Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer.J Gastrointest Cancer. 2021 Mar;52(1):23-30. doi: 10.1007/s12029-020-00518-5. Epub 2020 Sep 14. J Gastrointest Cancer. 2021. PMID: 32926335 Free PMC article. Review.
-
T Cell Cytokines Impact Epithelial Cell Responses during Helicobacter pylori Infection.J Immunol. 2020 Mar 15;204(6):1421-1428. doi: 10.4049/jimmunol.1901307. J Immunol. 2020. PMID: 32152211 Free PMC article. Review.
References
-
- Acio-Pizzarello CR, Acio AA, Choi EJ et al. . Determinants of the regulation of H. pylori adhesins include repeat sequences in both promoter and coding regions as well as the two component system, ArsRS. J Med Microbiol 2017;66:798–807. - PubMed
-
- Chen J, Lin M, Li N et al. . Therapeutic vaccination with Salmonella-derived codon optimized outer inflammatory protein DNA vaccine enhances protection in Helicobacter pylori infected mice. Vaccine 2012;30:5310–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

