Navigation-Linked Heads-Up Display in Intracranial Surgery: Early Experience
- PMID: 29040677
- PMCID: PMC6047456
- DOI: 10.1093/ons/opx205
Navigation-Linked Heads-Up Display in Intracranial Surgery: Early Experience
Abstract
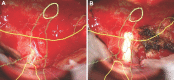
Background: The use of intraoperative navigation during microscope cases can be limited when attention needs to be divided between the operative field and the navigation screens. Heads-up display (HUD), also referred to as augmented reality, permits visualization of navigation information during surgery workflow.
Objective: To detail our initial experience with HUD.
Methods: We retrospectively reviewed patients who underwent HUD-assisted surgery from April 2016 through April 2017. All lesions were assessed for accuracy and those from the latter half of the study were assessed for utility.
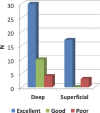
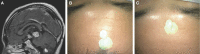
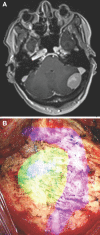
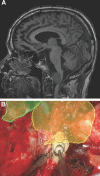
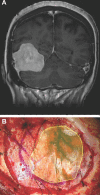
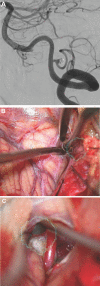
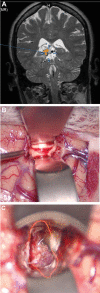
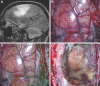
Results: Seventy-nine patients with 84 pathologies were included. Pathologies included aneurysms (14), arteriovenous malformations (6), cavernous malformations (5), intracranial stenosis (3), meningiomas (27), metastasis (4), craniopharygniomas (4), gliomas (4), schwannomas (3), epidermoid/dermoids (3), pituitary adenomas (2) hemangioblastoma (2), choroid plexus papilloma (1), lymphoma (1), osteoblastoma (1), clival chordoma (1), cerebrospinal fluid leak (1), abscess (1), and a cerebellopontine angle Teflon granuloma (1). Fifty-nine lesions were deep and 25 were superficial. Structures identified included the lesion (81), vessels (48), and nerves/brain tissue (31). Accuracy was deemed excellent (71.4%), good (20.2%), or poor (8.3%). Deep lesions were less likely to have excellent accuracy (P = .029). HUD was used during bed/head positioning (50.0%), skin incision (17.3%), craniotomy (23.1%), dural opening (26.9%), corticectomy (13.5%), arachnoid opening (36.5%), and intracranial drilling (13.5%). HUD was deactivated at some point during the surgery in 59.6% of cases. There were no complications related to HUD use.
Conclusion: HUD can be safely used for a wide variety of vascular and oncologic intracranial pathologies and can be utilized during multiple stages of surgery.
Figures




Comment in
-
In Reply: Navigation-Linked Heads-Up Display in Intracranial Surgery: Early Experience.Oper Neurosurg. 2018 Jun 1;14(6):E73. doi: 10.1093/ons/opy049. Oper Neurosurg. 2018. PMID: 29590451 No abstract available.
-
Letter: Navigation-Linked Heads-Up Display in Intracranial Surgery: Early Experience.Oper Neurosurg. 2018 Jun 1;14(6):E71-E72. doi: 10.1093/ons/opy048. Oper Neurosurg. 2018. PMID: 29590481 No abstract available.
References
-
- Yoon JW, Chen RE, Han PK, Si P, Freeman WD, Pirris SM. Technical feasibility and safety of an intraoperative head-up display device during spine instrumentation. Int J Med Robot. 2016. doi:10.1002/rcs.1770. - PubMed
-
- Eckardt C, Paulo EB. Heads-Up surgery for vitreoretinal procedures Retina. 2016;36(1):137-147. - PubMed
-
- Chimenti PC, Mitten DJ. Google glass as an alternative to standard fluoroscopic visualization for percutaneous fixation of hand fractures Plast Reconstr Surg. 2015;136(2):328-330. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

