Inhaled versus systemic corticosteroids for preventing bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates
- PMID: 29041034
- PMCID: PMC6485718
- DOI: 10.1002/14651858.CD002058.pub3
Inhaled versus systemic corticosteroids for preventing bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates
Abstract
Background: Bronchopulmonary dysplasia (BPD) remains an important cause of mortality and morbidity in preterm infants and inflammation plays a significant role in its pathogenesis. The use of inhaled corticosteroids may modulate the inflammatory process without concomitant high systemic steroid concentrations and less risk of adverse effects. This is an update of a review published in 2012 (Shah 2012). We recently updated the related review on "Inhaled versus systemic corticosteroids for treating bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates".
Objectives: To determine the effect of inhaled versus systemic corticosteroids started within the first 7 days of life on preventing death or BPD in ventilated very low birth weight infants.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 1), MEDLINE via PubMed (1966 to 23 February 2017), Embase (1980 to 23 February 2017), and CINAHL (1982 to 23 February 2017). We searched clinical trials registers, conference proceedings and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi-randomised trials.
Selection criteria: Randomised or quasi-randomised controlled trials comparing inhaled versus systemic corticosteroid therapy (irrespective of dose and duration) starting in the first seven days of life in very low birth weight preterm infants receiving assisted ventilation.
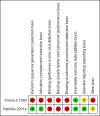
Data collection and analysis: Clinical outcomes data were extracted and analysed using Review Manager. When appropriate, meta-analysis was performed using typical relative risk (RR), typical risk difference (RD) and weighted mean difference (WMD). Meta-analyses were performed using typical relative risk, typical risk difference (RD), and weighted mean difference with their 95% confidence intervals (CI). If RD was statistically significant, the number needed to benefit or the number needed to harm was calculated. We assessed the quality of evidence was evaluated using GRADE principles.
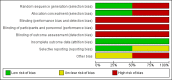
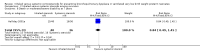
Main results: We included two trials that involved 294 infants. No new studies were included for the 2017 update. The incidence of death or BPD at 36 weeks' postmenstrual age was not statistically significantly different between infants who received inhaled or systemic steroids (RR 1.09, 95% CI 0.88 to 1.35; RD 0.05, 95% CI -0.07 to 0.16; 1 trial, N = 278). The incidence of BPD at 36 weeks' postmenstrual age among survivors was not statistically significant between groups (RR 1.34, 95% CI 0.94 to 1.90; RD 0.11, 95% CI -0.02 to 0.24; 1 trial, N = 206). There was no statistically significant difference in the outcomes of BPD at 28 days, death at 28 days or 36 weeks' postmenstrual age and the combined outcome of death or BPD by 28 days between groups (2 trials, N = 294). The duration of mechanical ventilation was significantly longer in the inhaled steroid group compared with the systemic steroid group (typical MD 4 days, 95% CI 0.2 to 8; 2 trials, N = 294; I² = 0%) as was the duration of supplemental oxygen (typical MD 11 days, 95% CI 2 to 20; 2 trials, N = 294; I² = 33%).The incidence of hyperglycaemia was significantly lower with inhaled steroids (RR 0.52, 95% CI 0.39 to 0.71; RD -0.25, 95% CI -0.37 to -0.14; 1 trial, N = 278; NNTB 4, 95% CI 3 to 7 to avoid 1 infant experiencing hyperglycaemia). The rate of patent ductus arteriosus increased in the group receiving inhaled steroids (RR 1.64, 95% CI 1.23 to 2.17; RD 0.21, 95% CI 0.10 to 0.33; 1 trial, N = 278; NNTH 5, 95% CI 3 to 10). In a subset of surviving infants in the United Kingdom and Ireland there were no significant differences in developmental outcomes at 7 years of age. However, there was a reduced risk of having ever been diagnosed as asthmatic by 7 years of age in the inhaled steroid group compared with the systemic steroid group (N = 48) (RR 0.42, 95% CI 0.19 to 0.94; RD -0.31, 95% CI -0.58 to -0.05; NNTB 3, 95% CI 2 to 20).According to GRADE the quality of the evidence was moderate to low. Evidence was downgraded on the basis of design (risk of bias), consistency (heterogeneity) and precision of the estimates.Both studies received grant support and the industry provided aero chambers and metered dose inhalers of budesonide and placebo for the larger study. No conflict of interest was identified.
Authors' conclusions: We found no evidence that early inhaled steroids confer important advantages over systemic steroids in the management of ventilator-dependent preterm infants. Based on this review inhaled steroids cannot be recommended over systemic steroids as a part of standard practice for ventilated preterm infants. Because they might have fewer adverse effects than systemic steroids, further randomised controlled trials of inhaled steroids are needed that address risk/benefit ratio of different delivery techniques, dosing schedules and long-term effects, with particular attention to neurodevelopmental outcome.
Conflict of interest statement
Dr Sachin S Shah has no conflict of interest to declare.
Dr Arne Ohlsson has no conflict of interest to declare.
Dr Henry L Halliday is the first author of an included trial (Halliday 2001a).
Dr. Vibhuti S Shah has no conflict of interest to declare.
Figures































Update of
-
Inhaled versus systemic corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates.Cochrane Database Syst Rev. 2012 May 16;(5):CD002058. doi: 10.1002/14651858.CD002058.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Oct 17;10:CD002058. doi: 10.1002/14651858.CD002058.pub3. PMID: 22592683 Updated.
References
References to studies included in this review
Groneck 1999 {published data only}
-
- Groneck P, Goetze‐Speer B, Speer CP. Effects of inhaled beclomethasone compared to systemic dexamethasone on lung inflammation in preterm infants at risk of chronic lung disease. Pediatric Pulmonology 1999;27(6):383‐7. [PUBMED: 10380089] - PubMed
Halliday 2001a {published data only}
-
- Halliday HL, Patterson CC, Halahakoon CWNL, European Multicenter Steroid Study Group. A multicentre, randomized open study of early corticosteroid treatment (OSECT) in preterm infants with respiratory illness: comparison of early and late treatment and of dexamethasone and inhaled budesonide. Pediatrics 2001;107(2):232‐40. - PubMed
-
- Wilson TT. Long term neurodevelopmental and psychosocial outcomes following premature birth: Has postnatal corticosteroid treatment been an over‐looked factor? [PhD Thesis]. School of Psychology, Faculty of Science & Agriculture. The Queen’s University of Belfast, 2005.
-
- Wilson TT, Waters L, Patterson CC, McCusker CG, Rooney NM, Marlow N, et al. Neurodevelopmental and respiratory follow‐up results at 7 Years for children from the United Kingdom and Ireland enrolled in a randomized trial of early and late postnatal corticosteroid treatment, systemic and inhaled (the Open Study of Early Corticosteroid Treatment). Pediatrics 2006;117(6):2196‐205. [DOI: 10.1542/peds.2005-2194; PUBMED: 16740865] - DOI - PubMed
References to studies excluded from this review
Dimitriou 1997 {published data only}
-
- Dimitriou G, Greenough A, Giffin FJ, Kavadia V. Inhaled versus systemic steroids in chronic oxygen dependency of preterm infants. European Journal of Pediatrics 1997;156(1):51‐5. [PUBMED: 9007492] - PubMed
Kovács 1998 {published data only}
-
- Kovács L, Davis GM, Faucher D, Papageorgiou A. Efficacy of sequential early systemic and inhaled corticosteroid therapy in the prevention of chronic lung disease of prematurity. Acta Paediatrica 1998;87(7):792‐8. [PUBMED: 9722255] - PubMed
Mazulov 2013 {published data only}
-
- Mazulov O, Yablon O, Bertsun K, Vzhetson E. Corticosteroids for preventing chronic lung disease in very low birth weight preterm neonates ‐ are they useful?. European Respiratory Journal 2013;42(Suppl 57):5126. [erj.ersjournals.com/content/erj/42/Suppl_57/P2057.full.pdf]
Parikh 2004 {published data only}
-
- Parikh NA, Locke RG, Chidekel A, Leef KH, Emberger J, Paul DA, et al. Effect of inhaled corticosteroid on markers of pulmonary inflammation and lung maturation in preterm infants with evolving chronic lung disease. Journal of the American Osteopathic Association 2004;104(3):114‐20. [PUBMED: 15083986] - PubMed
Additional references
Aghajafari 2001
Arias‐Camison 1999
-
- Arias‐Camison JM, Lau J, Cole CH, Frantz ID 3rd. Meta‐analysis of dexamethasone therapy started in the first 15 days of life for prevention of chronic lung disease in premature infants. Pediatric Pulmonology 1999;28(3):167‐74. [PUBMED: 10495332] - PubMed
Arnon 1992
Bahadue 2012
Bassler 2015
Bell 1978
Bhuta 1998
Clyman 1981
-
- Clyman RI, Mauray F, Roman C, Rudolf AM, Heymann MA. Glucocorticoids alter the sensitivity of the lamb ductus arteriosus to prostaglandin E2. Journal of Pediatrics 1981;98(1):126‐8. [PUBMED: 7452389] - PubMed
Clyman 1987
-
- Clyman RI. Ductus arteriosus: current theories of prenatal and postnatal regulation. Seminars in Perinatology 1987;11(1):67‐71. - PubMed
Davis 2001
Doyle 2014a
Doyle 2014b
Garland 1999
-
- Garland JS, Alex CP, Pauly TH, Whitehead VL, Brand J, Winston JF, et al. A three day course of dexamethasone therapy to prevent chronic lung disease in ventilated neonates: a randomised trial. Pediatrics 1999;104(1):91‐9. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 23 February 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grigg 1992
Groneck 1994
-
- Groneck P, Goetze‐Speer B, Opperman M, Eiffert H, Speer CP. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high risk preterm neonates. Pediatrics 1994;93(5):712‐8. [PUBMED: 8165067] - PubMed
Gupta 2000
-
- Gupta GK, Cole CH, Abbasi S, Demissie S, Njinimbam C, Nielsen HC, et al. Effects of early inhaled beclomethasone therapy on tracheal aspirate inflammatory mediators IL‐8 and IL‐1ra in ventilated preterm infants at risk for bronchopulmonary dysplasia. Pediatric Pulmonology 2000;30(4):275‐81. [PUBMED: 11015126] - PubMed
Halliday 1999
-
- Halliday HL. Clinical trials of postnatal corticosteroids: inhaled and systemic. Biology of the Neonate 1999;76(Suppl 1):29‐40. [DOI: ; PUBMED: 10393391] - PubMed
Halliday 2001b
-
- Halliday HL. Guidelines on neonatal steroids. Prenatal and Neonatal Medicine 2001;6(6):371‐3.
Heyman 1990
-
- Heyman E, Ohlsson A, Shennan AT, Heilbut M, Coceani F. Closure of patent ductus arteriosus after treatment with dexamethasone. Acta Paediatrica Scandinavica 1990;79(6‐7):698‐700. [PUBMED: 2386065] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horbar 1993
-
- Horbar JD, Wright EC, Onstad L, National Institute of Child Health and Human Development Neonatal Research Network. Decreasing mortality associated with introduction of surfactant therapy: an observational study of neonates weighing 601 to 1300 grams at birth. Pediatrics 1993;92(2):191‐6. [PUBMED: 7710456] - PubMed
Ibrahim 2011
ICROP 1984
-
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Archives of Ophthalmology 1984;102(8):1130‐4. [PUBMED: 6547831] - PubMed
Jefferies 2012
Kotecha 1996
-
- Kotecha S, Wangoo A, Silverman M, Shaw RJ. Increase in the concentrations of transforming growth factor beta‐1 in bronchoalveolar lavage fluid before development of chronic lung disease of prematurity. Journal of Pediatrics 1996;128(4):464‐9. [PUBMED: 8618178] - PubMed
Lee 2000
-
- Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU Network: 1996‐1997. Pediatrics 2000;106(5):1070‐9. [PUBMED: 11061777] - PubMed
Ng 1993
O'Callaghan 1992
Onland 2017a
Onland 2017b
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weight less than 1500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] - PubMed
Pierce 1995
-
- Pierce MR, Bancalari E. The role of inflammation in the pathogenesis of bronchopulmonary dysplasia. Pediatric Pulmonology 1995;19(6):371‐8. [PUBMED: 7567218] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Roberts 2017
Saarela 1999
-
- Saarela T, Vaarala A, Lanning P, Koivisto M. Incidence, ultrasonic patterns and resolution of nephrocalcinosis in very low birth weight infants. Acta Paediatrica 1999;88(6):655‐60. [PUBMED: 10419252] - PubMed
Schwartz 1994
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. gdt.gradepro.org/app/handbook/handbook.html. accessed prior to 12 September 2017.
Shah 2001
-
- Shah V, Ohlsson A. Postnatal dexamethasone in the prevention of chronic lung disease. Recent Advances in Paediatrics 2001;19:77‐96.
Shah 2017a
Shah 2017b
Shaw 1993
-
- Shaw NJ, Gill AB, Weindling AM, Cooke RW. The changing incidence of chronic lung disease. Health Trends 1993;25(2):50‐3. [PUBMED: 10130806] - PubMed
Shinwell 2000
Shinwell 2016
Sinkin 1990
-
- Sinkin RA, Cox C, Phelps DL. Predicting risk for bronchopulmonary dysplasia: selection criteria for clinical trials. Pediatrics 1990;86(5):728‐36. [PUBMED: 2235227] - PubMed
Soll 1999
-
- Soll RF, The Vermont Oxford Network Steroid Study Group. Early dexamethasone therapy for the prevention of chronic lung disease. Pediatric Research 1999;45:226A. [DOI: 10.1203/00006450-199904020-01345] - DOI
Soll 2002
Speer 1993
-
- Speer CP, Ruess D, Harms K, Herting E, Gefeller O. Neutrophil elastase and acute pulmonary damage in neonates with severe respiratory distress syndrome. Pediatrics 1993;91(4):794‐9. [PUBMED: 8464669] - PubMed
Stark 2001
-
- Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran EF, et al. National Institute of Child Health and Human Development Neonatal Research Network. Adverse effects of early dexamethasone treatment in extremely‐low‐birth‐weight infants. New England Journal of Medicine 2001;344(2):95‐101. [DOI: 10.1056/NEJM200101113440203; PUBMED: 11150359] - DOI - PubMed
Ward 2003
Watterberg 1994
-
- Watterberg KL, Carmichael DF, Gerdes JS, Werner S, Backstorm C, Murphy S. Secretory leukocyte protease inhibitor and lung inflammation in developing bronchopulmonary dysplasia. Journal of Pediatrics 1994;125(2):264‐9. [PUBMED: 7913723] - PubMed
Watterberg 1996
-
- Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97(2):210‐5. [PUBMED: 8584379] - PubMed
Watterberg 2012
Watts 1992
-
- Watts CL, Fanaroff AA, Bruce MC. Elevation of fibronectin levels in lung secretions of infants with respiratory distress syndrome and development of bronchopulmonary dysplasia. Journal of Pediatrics 1992;120(4 Pt 1):614‐20. [PUBMED: 1552403] - PubMed
Yeh 1997
-
- Yeh TF, Lin YJ, Hsieh WS, Lin HC, Lin CH, Chen JY, et al. Early postnatal dexamethasone therapy for prevention of chronic lung disease in preterm infants with respiratory distress syndrome: a multicentre clinical trial. Pediatrics 1997;100(4):e3. - PubMed
Yeh 1998
-
- Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow up study. Pediatrics 1998;101(5):E7. - PubMed
Zubrow 1995
-
- Zubrow AB, Hulman S, Kushner H, Falkner B, Philadelphia Neonatal Blood Pressure Study Group. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Journal of Perinatology 1995;15(6):470‐9. [PUBMED: 8648456] - PubMed
References to other published versions of this review
Shah 2003
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

