Societal preferences for adjuvant melanoma health states: UK and Australia
- PMID: 29041898
- PMCID: PMC5646133
- DOI: 10.1186/s12885-017-3673-y
Societal preferences for adjuvant melanoma health states: UK and Australia
Abstract
Background: No studies have measured preference-based utility weights for specific toxicities and outcomes associated with approved and investigational adjuvant treatments for patients with resected high-risk melanoma.
Methods: A cross-sectional study was conducted in the United Kingdom and Australia to obtain utilities for 14 adjuvant melanoma health states. One-on-one interviews were conducted using standard gamble; utility weights range from 0.0, dead, to 1.0, full health. Supplemental risk questions also were asked.
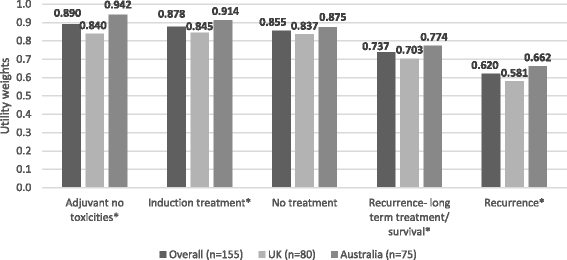
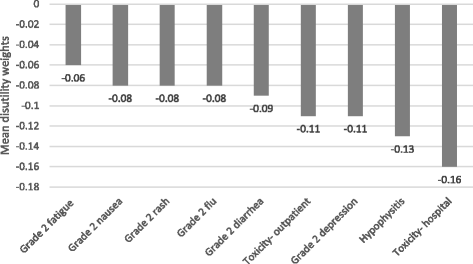
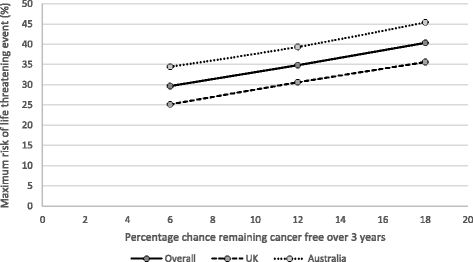
Results: Among 155 participants (52% male; mean age, 46 years) "adjuvant treatment no toxicities" (0.89) was most preferred, followed by "induction treatment" (0.88), and "no treatment" (0.86). Participants least preferred "cancer recurrence" (0.62); the utility for "cancer recurrence and 10-year survival with treatment" was 0.70. Disutilities for grade 2 toxicities ranged from -0.06 for fatigue to -0.13 for hypophysitis. The mean maximum acceptable risk of a life-threatening event ranged from 30% for a 6% increase in the chance of remaining cancer free over 3 years to 40% for an 18% increase; Australian respondents were willing to take higher risks.
Conclusion: Reproducible health utilities for adjuvant melanoma health states were obtained from the general population in two countries. These utilities can be incorporated into treatment-specific cost-effectiveness evaluations.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by Magil Institutional Review Board (Rockville, MD) and complied with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
MM: Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, Clovis, GlaxoSmithKline, Immunocore (uncompensated), Merck, Roche (all compensated); Research Funding: Amgen, AstraZeneca, Bristol-Myers Squibb, Clovis, Eisai, GlaxoSmithKline, Immunocore, Medimmune, Merck, Pfizer, Roche, Vertex; Travel, Accommodations, Expenses: Merck, Roche. MBA: Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, Caladrius, GlaxoSmithKline, Genentech Roche, Merck, Nektar, Novartis, Pfizer. KA: Consulting role: Bristol-Myers Squibb. PFW: Employment: Bristol-Myers Squibb. SK: Employment: Bristol-Myers Squibb; Stock or Other Ownership: Bristol-Myers Squibb; Research Funding: Bristol-Myers Squibb; Travel, Accommodations, Expenses: Bristol-Myers Squibb. JS: Employment: Bristol-Myers Squibb; Stock or Other Ownership: Bristol-Myers Squibb; Research Funding: Bristol-Myers Squibb. KB: Consulting role: Bristol-Myers Squibb.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Societal preference values for advanced melanoma health states in the United Kingdom and Australia.Br J Cancer. 2009 Aug 4;101(3):387-9. doi: 10.1038/sj.bjc.6605187. Epub 2009 Jul 14. Br J Cancer. 2009. PMID: 19603025 Free PMC article.
-
Cost-effectiveness of ipilimumab versus high-dose interferon as an adjuvant therapy in resected high-risk melanoma.Cancer Med. 2021 Oct;10(19):6618-6626. doi: 10.1002/cam4.4194. Epub 2021 Aug 17. Cancer Med. 2021. PMID: 34402192 Free PMC article.
-
Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma.N Engl J Med. 2017 Nov 9;377(19):1824-1835. doi: 10.1056/NEJMoa1709030. Epub 2017 Sep 10. N Engl J Med. 2017. PMID: 28891423 Clinical Trial.
-
Treatment of High Risk Resected Melanoma in Australia: Current Landscape and Practises.Australas J Dermatol. 2020 Aug;61(3):203-209. doi: 10.1111/ajd.13309. Epub 2020 May 13. Australas J Dermatol. 2020. PMID: 32403192 Review.
-
Preferences for Immunotherapy in Melanoma: A Systematic Review.Ann Surg Oncol. 2020 Feb;27(2):571-584. doi: 10.1245/s10434-019-07963-y. Epub 2019 Oct 29. Ann Surg Oncol. 2020. PMID: 31664622
Cited by
-
An Economic Evaluation of Pembrolizumab Versus Other Adjuvant Treatment Strategies for Resected High-Risk Stage III Melanoma in the USA.Clin Drug Investig. 2020 Jul;40(7):629-643. doi: 10.1007/s40261-020-00922-6. Clin Drug Investig. 2020. PMID: 32418051 Free PMC article.
References
-
- World Health Organization. http://www.who.int/uv/faq/skincancer/en/index1.html. Accessed 09 Dec 2015.
-
- Cancer Research UK. http://www.cancerresearchuk.org/health-professional/cancer-statistics/st.... Accessed 28 Sept 2015.
-
- Grob JJ, Jouary T, Dréno B, et al. Adjuvant therapy with pegylated interferon alfa-2b (36 months) versus low-dose interferon alfa-2b (18 months) in melanoma patients without macrometastatic nodes: an open-label, randomised, phase 3 European Association for Dermato-Oncology (EADO) study. Eur J Cancer. 2013;49(1):166–174. doi: 10.1016/j.ejca.2012.07.018. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical