Prevalence of drug-resistant pulmonary tuberculosis in India: systematic review and meta-analysis
- PMID: 29041901
- PMCID: PMC5645895
- DOI: 10.1186/s12889-017-4779-5
Prevalence of drug-resistant pulmonary tuberculosis in India: systematic review and meta-analysis
Abstract
Background: Drug-resistant pulmonary tuberculosis (DR-TB) is a significant public health issue that considerably deters the ongoing TB control efforts in India. The purpose of this review was to investigate the prevalence of DR-TB and understand the regional variation in resistance pattern across India from 1995 to 2015, based on a large body of published epidemiological studies.
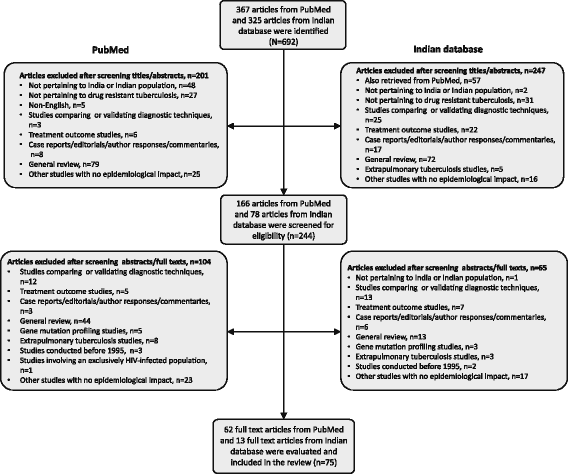
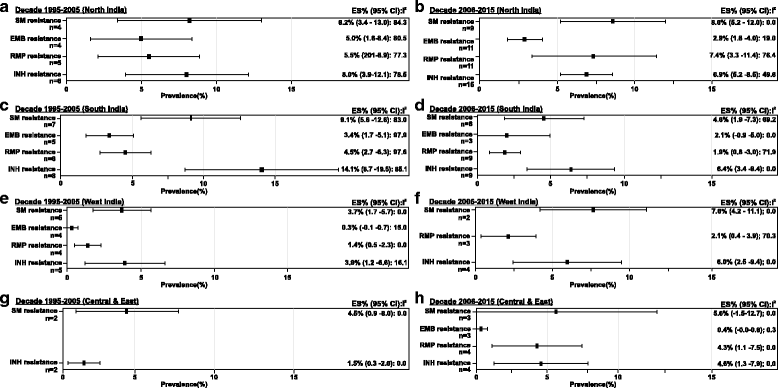
Methods: A systematic review of published studies reporting prevalence of DR-TB from biomedical databases (PubMed and IndMed) was conducted. Meta-analysis was performed using random effects model and the pooled prevalence estimate (95% confidence interval [CI]) of DR-TB, multidrug resistant (MDR-) TB, pre-extensively drug-resistant (pre-XDR) TB and XDR-TB were calculated across two study periods (decade 1: 1995 to 2005; decade 2: 2006 to 2015), countrywide and in different regions. Heterogeneity in this meta-analysis was assessed using I2 statistic.
Results: A total of 75 of 635 screened studies that fulfilled the inclusion criteria were selected. Over 40% of 45,076 isolates suspected for resistance to any first-line anti-TB drugs tested positive. Comparative analysis revealed a worsening trend in DR-TB between the two study decades (decade 1: 37.7% [95% CI = 29.0; 46.4], n = 25 vs decade 2: 46.1% [95% CI = 39.0; 53.2], n = 36). The pooled estimate of MDR-TB resistance was higher in previously treated patients (decade 1: 29.8% [95% CI = 20.7; 39.0], n = 13; decade 2: 35.8% [95% CI = 29.2; 42.4], n = 24) as compared with the newly diagnosed cases (decade 1: 4.1% [95% CI = 2.7; 5.6], n = 13; decade 2: 5.6% [95% CI = 3.8; 7.4], n = 17). Overall, studies from Western states of India reported highest prevalence of DR-TB (57.8% [95% CI = 37.4; 78.2], n = 6) and MDR-TB (39.9% [95% CI = 21.7; 58.0], n = 6) during decade 2. Prevalence of pre-XDR TB was 7.9% (95% CI = 4.4; 11.4, n = 5) with resistance to fluoroquinolone (66.3% [95% CI = 58.2; 74.4], n = 5) being the highest. The prevalence of XDR-TB was 1.9% (95% CI = 1.2; 2.6, n = 14) over the 20-year period.
Conclusion: The alarming increase in the trend of anti-TB drug resistance in India warrants the need for a structured nationwide surveillance to assist the National TB Control Program in strengthening treatment strategies for improved outcomes.
Keywords: Drug-resistant tuberculosis; India; Prevalence.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Drs. Goyal, Kadam, Narang and Singh are employees of Janssen India and hold company stocks. The authors declare that they have no other competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Multidrug resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: a systematic review and meta-analysis.BMC Infect Dis. 2017 Mar 20;17(1):219. doi: 10.1186/s12879-017-2323-y. BMC Infect Dis. 2017. PMID: 28320336 Free PMC article.
-
Drug resistance among students with pulmonary tuberculosis: a study based on screening in Henan, China.BMC Infect Dis. 2025 Aug 8;25(1):1002. doi: 10.1186/s12879-025-11408-1. BMC Infect Dis. 2025. PMID: 40781271 Free PMC article.
-
Treatment outcomes for multidrug-resistant tuberculosis under DOTS-Plus: a systematic review and meta-analysis of published studies.Infect Dis Poverty. 2017 Jan 17;6(1):7. doi: 10.1186/s40249-016-0214-x. Infect Dis Poverty. 2017. PMID: 28093078 Free PMC article.
-
Variation and risk factors of drug resistant tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis.BMC Public Health. 2015 Mar 25;15:291. doi: 10.1186/s12889-015-1614-8. BMC Public Health. 2015. PMID: 25880829 Free PMC article.
-
Pre-extensively drug-resistant and extensively drug-resistant tuberculosis in Latin America and the Caribbean: A systematic review and meta-analysis.Am J Infect Control. 2024 Mar;52(3):349-357. doi: 10.1016/j.ajic.2023.12.001. Epub 2023 Dec 6. Am J Infect Control. 2024. PMID: 38061402
Cited by
-
Starting an All-Oral Longer Regimen in a Primary Multidrug-Resistant Pulmonary Tuberculosis Patient at a District Tuberculosis Center for the First Time: A Rare Case.Cureus. 2022 Jul 22;14(7):e27146. doi: 10.7759/cureus.27146. eCollection 2022 Jul. Cureus. 2022. PMID: 36004032 Free PMC article.
-
Prevalence and patterns of drug resistance among pulmonary tuberculosis patients in Hangzhou, China.Antimicrob Resist Infect Control. 2018 May 2;7:61. doi: 10.1186/s13756-018-0348-7. eCollection 2018. Antimicrob Resist Infect Control. 2018. PMID: 29744042 Free PMC article.
-
Evaluating Tuberculosis and Drug Resistance in Serbia: A Ten-Year Experience from a Tertiary Center.Antibiotics (Basel). 2025 Mar 18;14(3):320. doi: 10.3390/antibiotics14030320. Antibiotics (Basel). 2025. PMID: 40149130 Free PMC article.
-
Genotypic analysis of drug-resistant tuberculosis in Ghana: Insights into pre-XDR and XDR-TB.PLoS One. 2025 May 20;20(5):e0323527. doi: 10.1371/journal.pone.0323527. eCollection 2025. PLoS One. 2025. PMID: 40392805 Free PMC article.
-
Increasing prevalence of resistance to second-line drugs among multidrug-resistant Mycobacterium tuberculosis isolates in Kuwait.Sci Rep. 2021 Apr 8;11(1):7765. doi: 10.1038/s41598-021-87516-0. Sci Rep. 2021. PMID: 33833390 Free PMC article.
References
-
- World Health Organization. The Global Tuberculosis Report: 2015. Geneva, Switzerland: WHO, 2015. http://www.who.int/tb/publications/global_report/gtbr2015_executive_summ.... Accessed 2016, Jan 18.
-
- World Health Organization. The Global Tuberculosis Report: 2016. Geneva, Switzerland: WHO, 2016. http://www.who.int/tb/publications/global_report/en/. Accessed 2017, Feb 9.
-
- Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. WHO Guidelines Approved by the Guidelines Review Committee: 2014 update. Geneva, Switzerland: WHO, 2014. http://www.who.int/tb/publications/pmdt_companionhandbook/en/. Accessed 2016, Jan 18.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

