Cost-effectiveness analysis of XELOX versus XELOX plus bevacizumab for metastatic colorectal cancer in a public hospital school
- PMID: 29041915
- PMCID: PMC5645838
- DOI: 10.1186/s12885-017-3679-5
Cost-effectiveness analysis of XELOX versus XELOX plus bevacizumab for metastatic colorectal cancer in a public hospital school
Abstract
Background: Metastatic colorectal cancer imposes a substantial burden on patients and society. Over the last years, progresses in the treatment have been made especially due to the introduction of monoclonal antibodies, such as bevacizumab which, on the other hand, has considerably increased the costs of treatment. We performed a cost-effectiveness analysis of bevacizumab plus XELOX in comparison with XELOX alone in metastatic colorectal cancer in first-line therapy, from the perspective of a public hospital school in Brazil.
Methods: This was a cost-effectiveness analysis performed by a decision tree and Markov models. Costs were expressed in local currency and outcomes were expressed in months of life gained. The model was constructed using the TreeAge Pro 2013® software.
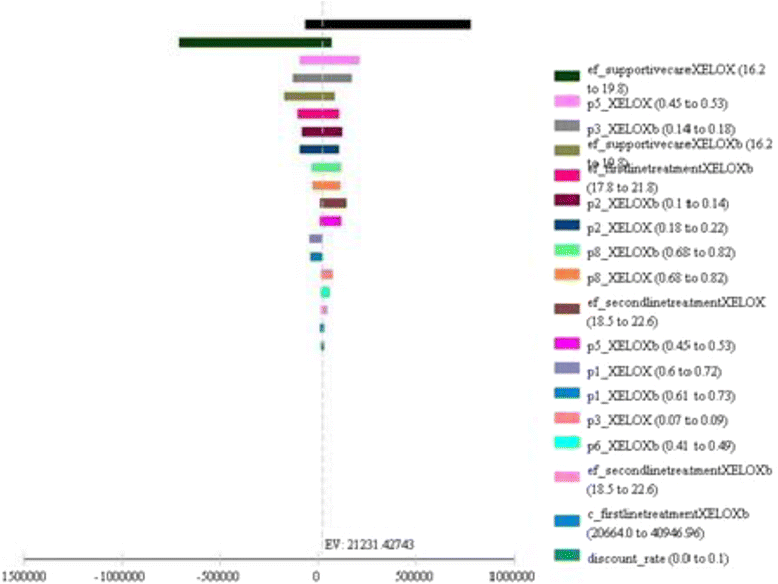
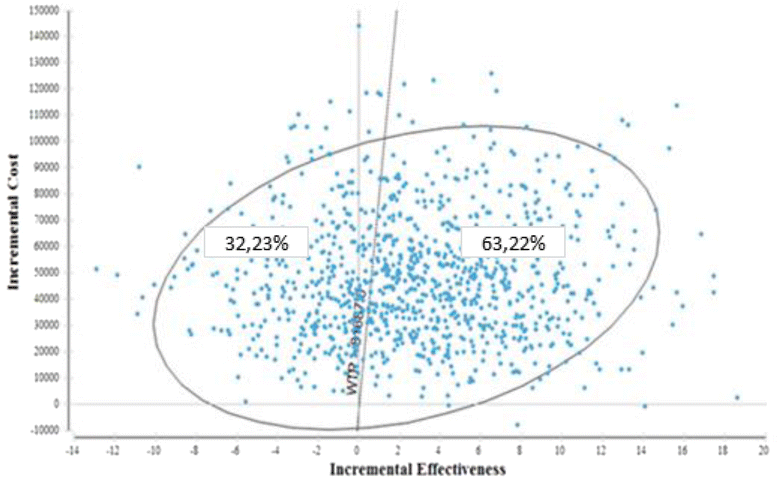
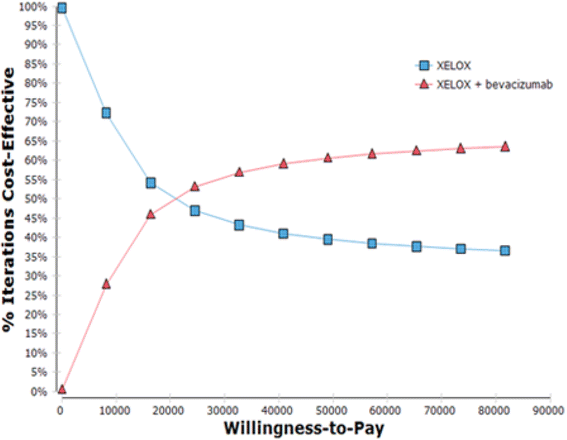
Results: The incremental difference in years of life gained was 2.25 months, with an extra cost of 47,833.57 BRL, resulting in an incremental cost-effectiveness of 21,231.43 BRL per month of life gained.
Conclusions: Although the XELOX plus bevacizumab regimen is a more expensive and more effective treatment than XELOX, it does not fit the reimbursement values fixed by the public healthcare system in Brazil.
Keywords: Bevacizumab; Brazil; Colorectal Neoplasms; Cost-effectiveness evaluation; Unified health system.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Medical School of Ribeirão Preto - University of São Paulo on April 17, 2013 (number 956/2013). The waiver of the written informed consent to patients was requested and approved in this study according to Resolution n°. 466/2012 of the Brazilian Ministry of Health.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Out-of-pocket payment and cost-effectiveness of XELOX and XELOX plus bevacizumab therapy: from the perspective of metastatic colorectal cancer patients in Japan.Int J Clin Oncol. 2010 Jun;15(3):256-62. doi: 10.1007/s10147-010-0045-x. Epub 2010 Mar 4. Int J Clin Oncol. 2010. PMID: 20198398
-
Cost-effectiveness analysis of colon cancer treatments from MOSIAC and No. 16968 trials.World J Gastroenterol. 2014 Dec 21;20(47):17976-84. doi: 10.3748/wjg.v20.i47.17976. World J Gastroenterol. 2014. PMID: 25548497 Free PMC article.
-
Cost-analysis of XELOX and FOLFOX4 for treatment of colorectal cancer to assist decision-making on reimbursement.BMC Cancer. 2011 Jul 9;11:288. doi: 10.1186/1471-2407-11-288. BMC Cancer. 2011. PMID: 21740590 Free PMC article.
-
Capecitabine, alone and in combination, in the management of patients with colorectal cancer: a review of the evidence.Drugs. 2008;68(7):949-61. doi: 10.2165/00003495-200868070-00005. Drugs. 2008. PMID: 18457461 Review.
-
Bevacizumab for metastatic colorectal cancer: a NICE single technology appraisal.Pharmacoeconomics. 2012 Dec 1;30(12):1119-32. doi: 10.2165/11597210-000000000-00000. Pharmacoeconomics. 2012. PMID: 23058097 Review.
Cited by
-
Treatment of Patients With Late-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline.JCO Glob Oncol. 2020 Mar;6:414-438. doi: 10.1200/JGO.19.00367. JCO Glob Oncol. 2020. PMID: 32150483 Free PMC article.
-
Economic Evaluation of Adding Bevacizumab to Chemotherapy for Metastatic Colorectal Cancer (mCRC) Patients in Indonesia.Asian Pac J Cancer Prev. 2021 Jun 1;22(6):1921-1926. doi: 10.31557/APJCP.2021.22.6.1921. Asian Pac J Cancer Prev. 2021. PMID: 34181352 Free PMC article.
-
Economic Evaluation of Monoclonal Antibodies in Metastatic Colorectal Cancer: A Systematic Review.Mol Diagn Ther. 2021 Nov;25(6):715-734. doi: 10.1007/s40291-021-00560-4. Epub 2021 Nov 24. Mol Diagn Ther. 2021. PMID: 34816395
References
-
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society, 2016.
-
- de Gramont A, Bosset JF, Milan C, Rougier P, Bouché O, Etienne PL, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15:808–815. doi: 10.1200/JCO.1997.15.2.808. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

