Diagnostic performance of the loop-mediated isothermal amplification (LAMP) based illumigene® malaria assay in a non-endemic region
- PMID: 29041927
- PMCID: PMC5645927
- DOI: 10.1186/s12936-017-2065-8
Diagnostic performance of the loop-mediated isothermal amplification (LAMP) based illumigene® malaria assay in a non-endemic region
Abstract
Background: Light microscopy and antigen-based rapid diagnostic tests are the primary diagnostic tools for detecting malaria, although being labour-intensive and frequently challenged by lack of personnel's experience and low levels of parasite density. The latter being especially important in non-endemic settings. Novel molecular techniques aim to overcome this drawback. The objective of this study was to assess the diagnostic performance of the illumigene malaria assay® (Meridian Bioscience) compared to microscopy, RDT and real-time PCR. This loop-mediated isothermal amplification (LAMP) assay is a qualitative in vitro diagnostic test for the direct detection of Plasmodium spp. DNA in human venous whole blood samples.
Methods: The illumigene assay was assessed on a retrospective panel of stored blood samples (n = 103) from returned travellers and external quality control samples (n = 12). Additionally the assay was prospectively assessed on 30 fresh routine samples with a request for malaria diagnosis. The illumigene assay was compared to microscopy, RDT and Plasmodium species specific real-time PCR.
Results: In the retrospective evaluation, the illumigene assay showed 100% agreement with the real-time PCR, RDT and microscopy yielding a sensitivity and specificity of 100% (95% CI 95.1-100% and 89.7-100%, respectively). Seven samples from patients recently treated for Plasmodium falciparum infection that were RDT positive and microscopy negative yielded positive test results. The performance of the illumigene assay equals that of microscopy combined with RDT in the prospective panel with three false negative RDT results and one false negative microscopy result. Excellent concordance with PCR was observed. The limit of detection of the assay approached 0.5 parasites/µL for both P. falciparum and Plasmodium vivax.
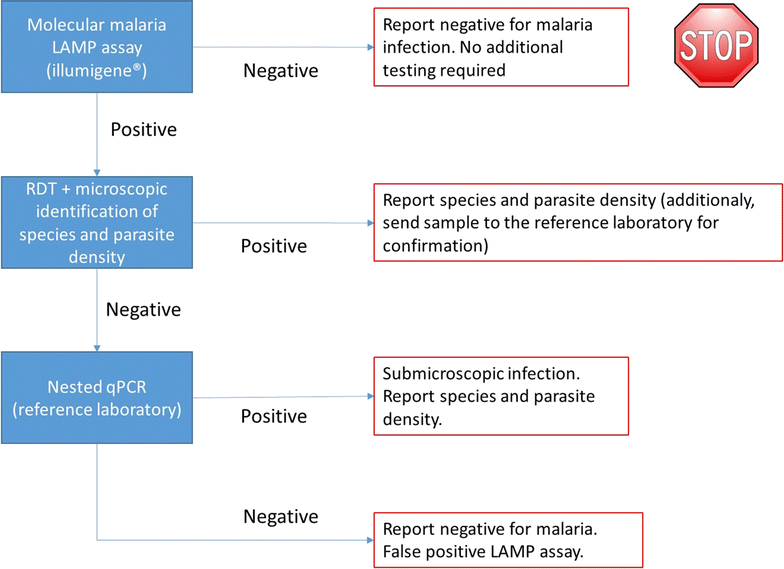
Conclusion: In non-endemic regions where the diagnostic process for malaria infections is questioned by lack of experience and low levels of parasite densities, the illumigene assay can be of value. Due to its high sensitivity, the LAMP assay may be considered as primary diagnostic test. The results of this study indicate that negative screen results do not need further confirmation. However, before implementation, this approach needs to be confirmed in larger, prospective studies. A shortcoming of this assay is that no species identification nor determination of parasite density are possible.
Keywords: Illumigene malaria assay; LAMP assay; Loop-mediated isothermal amplification; Malaria; Plasmodium.
Figures
References
-
- WHO. Policy brief on malaria diagnostics in low-transmission settings. Geneva: World Health Organization. http://www.who.int/malaria/publications/atoz/policy-brief-diagnosis-low-.... Accessed 1 Oct 2016.
-
- WHO. World Malaria Report 2016. Geneva: World Health Organization. http://www.who.int/malaria/publications/world-malaria-report-2016/report.... Accessed 27 Jan 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

