The Fear Reduction Exercised Early (FREE) approach to low back pain: study protocol for a randomised controlled trial
- PMID: 29041947
- PMCID: PMC5646107
- DOI: 10.1186/s13063-017-2225-8
The Fear Reduction Exercised Early (FREE) approach to low back pain: study protocol for a randomised controlled trial
Abstract
Background: Low back pain (LBP) is a major health issue associated with considerable health loss and societal costs. General practitioners (GPs) play an important role in the management of LBP; however, GP care has not been shown to be the most cost-effective approach unless exercise and behavioural counselling are added to usual care. The Fear Reduction Exercised Early (FREE) approach to LBP has been developed to assist GPs to manage LBP by empowering exploration and management of psychosocial barriers to recovery and provision of evidence-based care and information. The aim of the Low Back Pain in General Practice (LBPinGP) trial is to explore whether patients with LBP who receive care from GPs trained in the FREE approach have better outcomes than those who receive usual care.
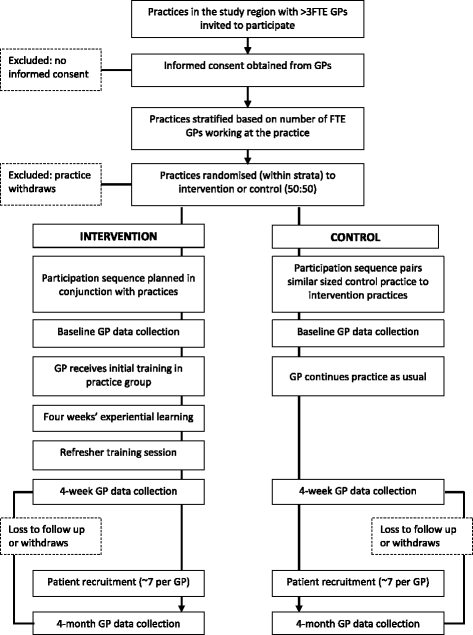
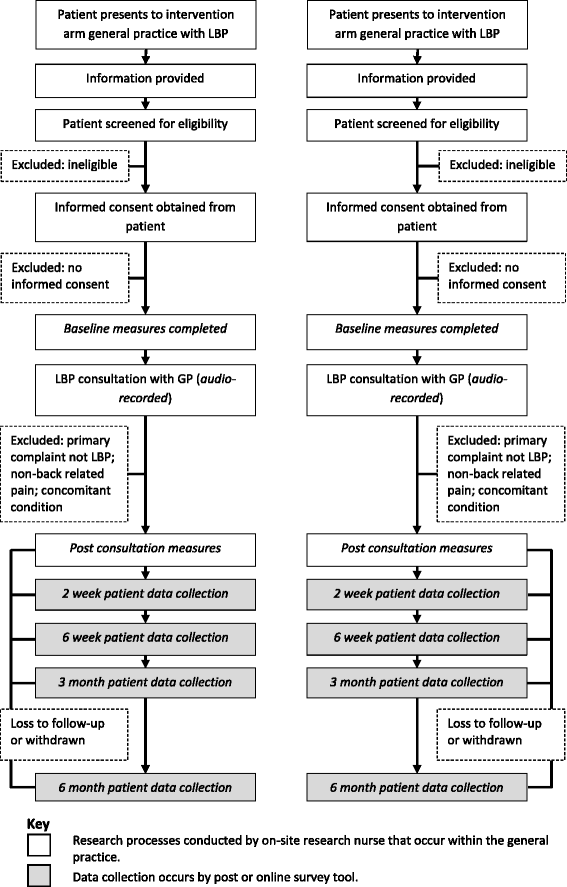
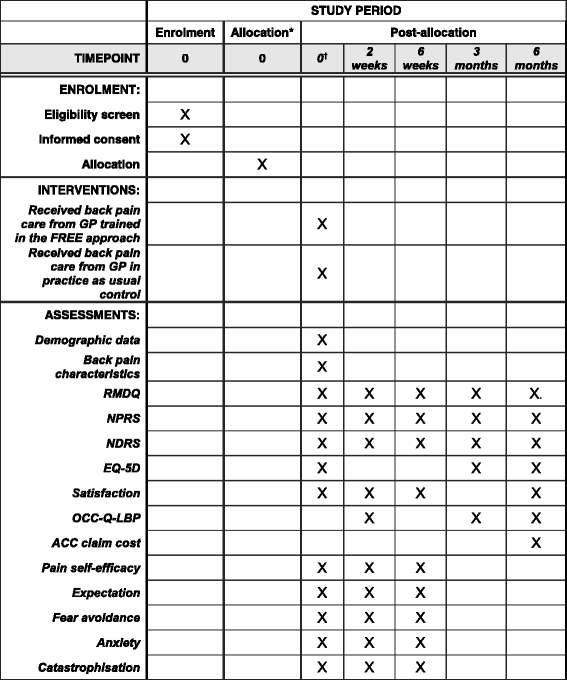
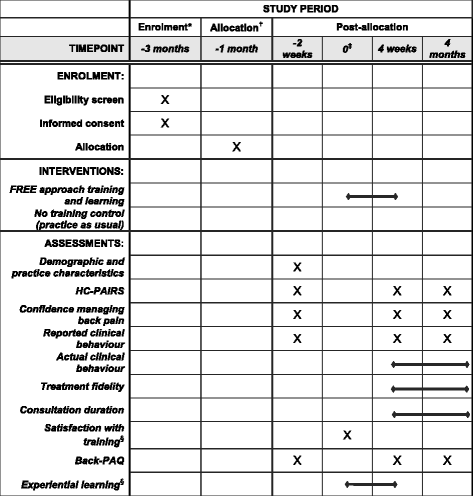
Methods/design: This is a cluster randomised controlled superiority trial comparing the FREE approach with usual care for LBP management with investigator-blinded assessment of outcomes. GPs will be recruited and then cluster randomised (in practice groups) to the intervention or control arm. Intervention arm GPs will receive training in the FREE approach, and control arm GPs will continue to practice as usual. Patients presenting to their GP with a primary complaint of LBP will be allocated on the basis of allocation of the GP they consult. We aim to recruit 60 GPs and 275 patients (assuming patients are recruited from 75% of GPs and an average of 5 patients per GP complete the study, accounting for 20% patient participant dropout). Patient participants and the trial statistician will be blind to group allocation throughout the study. Analyses will be undertaken on an intention-to-treat basis. The primary outcome will be back-related functional impairment 6 months post-initial LBP consultation (interim data at 2 weeks, 6 weeks and 3 months), measured with the Roland-Morris Disability Questionnaire. Secondary patient outcomes include pain, satisfaction, quality of life, days off from work and costs of care. Secondary GP outcomes include beliefs about pain and impairment, GP confidence, and actual and reported clinical behaviour. Health economic and process evaluations will be conducted.
Discussion: In the LBPinGP trial, we will investigate providing an intervention during the first interaction a person with back pain has with their GP. Because the FREE approach is used within a normal GP consultation, if effective, it may be a cost-effective means of improving LBP care.
Trial registration: Australian New Zealand Clinical Trials Registry, ACTRN12616000888460 . Registered on 6 July 2016.
Keywords: Brief intervention; Cost-effectiveness; General practice; Health-related quality of life; Impairment; Intervention study; Low back pain; Primary care; RCT; Treatment outcome.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the New Zealand Central Region Health and Disability Ethics Committee (16/CEN/43). All participants will provide written informed consent to participate. (
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
The Fear Reduction Exercised Early (FREE) approach to management of low back pain in general practice: A pragmatic cluster-randomised controlled trial.PLoS Med. 2019 Sep 9;16(9):e1002897. doi: 10.1371/journal.pmed.1002897. eCollection 2019 Sep. PLoS Med. 2019. PMID: 31498799 Free PMC article. Clinical Trial.
-
Comparing satisfaction with a participatory driven web-application and a standard website for patients with low back pain: a study protocol for a randomised controlled trial (part of the ADVIN Back Trial).Trials. 2018 Jul 25;19(1):399. doi: 10.1186/s13063-018-2795-0. Trials. 2018. PMID: 30045749 Free PMC article.
-
A multicentred randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain. The Back Skills Training (BeST) trial.Health Technol Assess. 2010 Aug;14(41):1-253, iii-iv. doi: 10.3310/hta14410. Health Technol Assess. 2010. PMID: 20807469 Clinical Trial.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.Health Technol Assess. 2005 Feb;9(3):iii-iv, 1-126. doi: 10.3310/hta9030. Health Technol Assess. 2005. PMID: 15694064 Review.
-
Core Stability Exercise Versus General Exercise for Chronic Low Back Pain.J Athl Train. 2017 Jan;52(1):71-72. doi: 10.4085/1062-6050-51.11.16. Epub 2016 Nov 16. J Athl Train. 2017. PMID: 27849389 Free PMC article. Review.
Cited by
-
A Guideline-Implementation Intervention to Improve the Management of Low Back Pain in Primary Care: A Difference-in-Difference-in-Differences Analysis.Appl Health Econ Health Policy. 2023 Mar;21(2):253-262. doi: 10.1007/s40258-022-00776-3. Epub 2022 Dec 6. Appl Health Econ Health Policy. 2023. PMID: 36471226 Free PMC article.
-
Patient education materials for non-specific low back pain and sciatica: A systematic review and meta-analysis.PLoS One. 2022 Oct 12;17(10):e0274527. doi: 10.1371/journal.pone.0274527. eCollection 2022. PLoS One. 2022. PMID: 36223377 Free PMC article.
-
The relationship between the psychological stress of adolescents in school and the prevalence of chronic low back pain: a cross-sectional study in China.Child Adolesc Psychiatry Ment Health. 2019 Jun 17;13:24. doi: 10.1186/s13034-019-0283-2. eCollection 2019. Child Adolesc Psychiatry Ment Health. 2019. PMID: 31236133 Free PMC article.
-
Models of care for managing non-specific low back pain.Cochrane Database Syst Rev. 2025 Mar 7;3(3):CD015083. doi: 10.1002/14651858.CD015083.pub2. Cochrane Database Syst Rev. 2025. PMID: 40052535
-
The Fear Reduction Exercised Early (FREE) approach to management of low back pain in general practice: A pragmatic cluster-randomised controlled trial.PLoS Med. 2019 Sep 9;16(9):e1002897. doi: 10.1371/journal.pmed.1002897. eCollection 2019 Sep. PLoS Med. 2019. PMID: 31498799 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

