Microparticles from patients with systemic lupus erythematosus induce production of reactive oxygen species and degranulation of polymorphonuclear leukocytes
- PMID: 29041967
- PMCID: PMC5646131
- DOI: 10.1186/s13075-017-1437-3
Microparticles from patients with systemic lupus erythematosus induce production of reactive oxygen species and degranulation of polymorphonuclear leukocytes
Abstract
Background: The interaction of circulating microparticles (MPs) with immune cells in systemic lupus erythematosus (SLE) is sparsely investigated. We examined the ability of MPs from SLE patients to induce production of reactive oxygen species (ROS) and degranulation of polymorphonuclear leukocytes (PMNs).
Methods: Plasma MPs, leukocytes and sera isolated from 20 SLE patients and 10 healthy controls were mixed in different combinations, with or without lipopolysaccharide (LPS), and incubated for 30 min. Dihydrorhodamine 123 was used to measure ROS production by flow cytometry. The ability of immunoglobulin G (IgG) isolated from five SLE patients to increase MP-induced production of ROS by PMNs was tested. Cell supernatants were analysed for content of primary, secondary and tertiary granule components by Luminex assays.
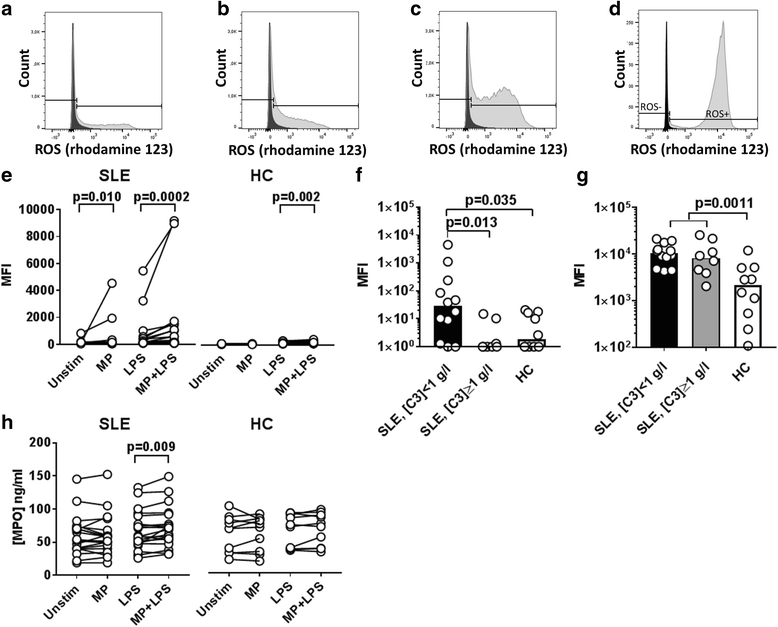
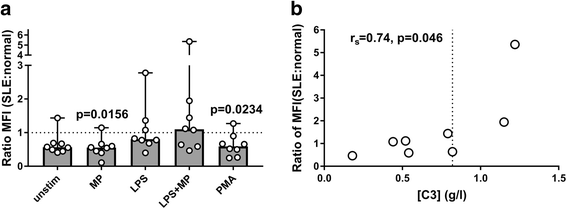
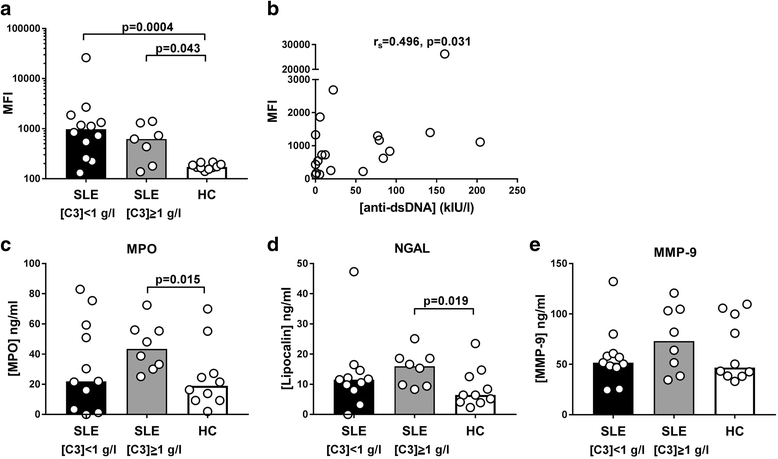
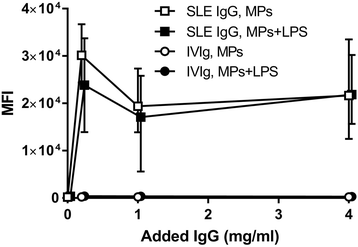
Results: MPs from SLE patients promoted ROS production by PMNs, and enhanced LPS-induced ROS production and release of primary granules by PMNs, when added to samples of autologous leukocytes and serum. In a similar autologous setting, MPs from healthy controls enhanced LPS-induced ROS production by PMNs. When leukocytes from a healthy control were stimulated with autologous MPs in the presence of various sera, SLE patient serum promoted ROS production and release of primary and secondary granules by PMNs. A role for antibodies in this respect was indicated by the observation that supplementation of normal serum with IgG from SLE patients promoted MP-induced ROS production by healthy PMNs. Moreover, when various MPs were incubated with leukocytes and serum from a healthy control, patient-derived MPs induced more ROS production by PMNs than did healthy control-derived MPs.
Conclusions: SLE patients display increased ROS production and degranulation by PMNs in response to MPs, which partly depends on serum components, including antibodies, MP properties and hyper-responsiveness of the PMNs per se.
Conflict of interest statement
Ethics approval and consent to participate
The Danish Regional Scientific Ethics Committee approved and consented to this study (protocol no. H-1-2013-046). We
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation.Arthritis Rheum. 2012 Apr;64(4):1227-36. doi: 10.1002/art.34381. Epub 2012 Jan 11. Arthritis Rheum. 2012. PMID: 22238051
-
Potential Involvement of Platelet-Derived Microparticles and Microparticles Forming Immune Complexes during Monocyte Activation in Patients with Systemic Lupus Erythematosus.Front Immunol. 2018 Mar 1;9:322. doi: 10.3389/fimmu.2018.00322. eCollection 2018. Front Immunol. 2018. PMID: 29545790 Free PMC article. Clinical Trial.
-
Circulating microparticles in systemic Lupus Erythematosus.Dan Med J. 2012 Nov;59(11):B4548. Dan Med J. 2012. PMID: 23171755 Review.
-
Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus.Arthritis Rheum. 2003 Oct;48(10):2888-97. doi: 10.1002/art.11237. Arthritis Rheum. 2003. PMID: 14558095
-
Cross-Talk among Polymorphonuclear Neutrophils, Immune, and Non-Immune Cells via Released Cytokines, Granule Proteins, Microvesicles, and Neutrophil Extracellular Trap Formation: A Novel Concept of Biology and Pathobiology for Neutrophils.Int J Mol Sci. 2021 Mar 18;22(6):3119. doi: 10.3390/ijms22063119. Int J Mol Sci. 2021. PMID: 33803773 Free PMC article. Review.
Cited by
-
Extracellular Vesicles Tune the Immune System in Renal Disease: A Focus on Systemic Lupus Erythematosus, Antiphospholipid Syndrome, Thrombotic Microangiopathy and ANCA-Vasculitis.Int J Mol Sci. 2021 Apr 18;22(8):4194. doi: 10.3390/ijms22084194. Int J Mol Sci. 2021. PMID: 33919576 Free PMC article. Review.
-
DNA as a self-antigen: nature and regulation.Curr Opin Immunol. 2018 Dec;55:31-37. doi: 10.1016/j.coi.2018.09.009. Epub 2018 Sep 24. Curr Opin Immunol. 2018. PMID: 30261321 Free PMC article. Review.
-
Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O.Front Immunol. 2021 Mar 4;12:649693. doi: 10.3389/fimmu.2021.649693. eCollection 2021. Front Immunol. 2021. PMID: 33746988 Free PMC article. Review.
-
Extracellular vesicles in Inflammatory Skin Disorders: from Pathophysiology to Treatment.Theranostics. 2020 Aug 7;10(22):9937-9955. doi: 10.7150/thno.45488. eCollection 2020. Theranostics. 2020. PMID: 32929326 Free PMC article. Review.
-
Extracellular Vesicles as Potential Theranostic Platforms for Skin Diseases and Aging.Pharmaceutics. 2021 May 20;13(5):760. doi: 10.3390/pharmaceutics13050760. Pharmaceutics. 2021. PMID: 34065468 Free PMC article. Review.
References
-
- Pereira J, Alfaro G, Goycoolea M, Quiroga T, Ocqueteau M, Massardo L, et al. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb Haemost. 2006;95:94–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

