Fluorescence optical imaging in pediatric patients with inflammatory and non-inflammatory joint diseases: a comparative study with ultrasonography
- PMID: 29041986
- PMCID: PMC5646108
- DOI: 10.1186/s13075-017-1440-8
Fluorescence optical imaging in pediatric patients with inflammatory and non-inflammatory joint diseases: a comparative study with ultrasonography
Abstract
Background: Valid detection of arthritis is essential in differential diagnosis of joint pain. Indocyanin green (ICG)-enhanced fluorescence optical imaging (FOI) is a new imaging method that visualizes inflammation in wrist and finger joints. Objectives of this study were to compare FOI with ultrasonography (US, by gray-scale (GS) and power Doppler (PD)) and clinical examination (CE) and to estimate the predictive power of FOI for discrimination between inflammatory and non-inflammatory juvenile joint diseases.
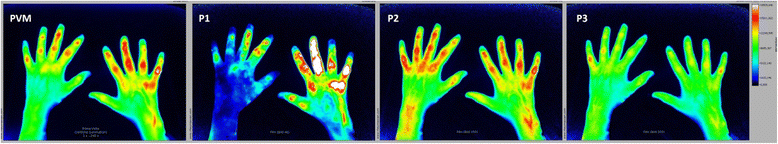
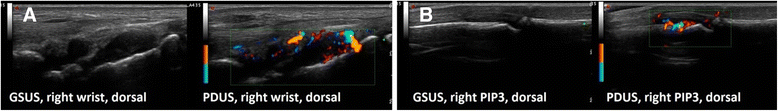
Methods: FOI and GSUS/PDUS were performed in both hands of 76 patients with joint pain (53 with juvenile idiopathic arthritis (JIA), 23 with non-inflammatory joint diseases). Inflammation was graded by a semiquantitative score (grades 0-3) for each imaging method. Joints were defined clinically active if swollen or tender with limited range of motion. Sensitivity and specificity of FOI in three phases dependent on ICG enhancement (P1-P3) were analyzed with CE and GSUS/PDUS as reference.
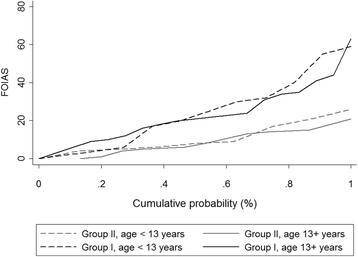
Results: For JIA patients, FOI had an overall sensitivity of 67.3%/72.0% and a specificity of 65.0%/58.8% with GSUS/PDUS as reference; specificity was highest in P3 (GSUS 94.3%/PDUS 91.7%). FOI was more sensitive for detecting clinically active joints than GSUS/PDUS (75.2% vs 57.3%/32.5%). In patients with non-inflammatory joint diseases both FOI and US showed positive (i.e., pathological) findings (25% and 14% of joints). The predictive value for discrimination between inflammatory and non-inflammatory joint diseases was 0.79 for FOI and 0.80/0.85 for GSUS/PDUS.
Conclusions: Dependent on the phase evaluated, FOI had moderate to good agreement with CE and US. Both imaging methods revealed limitations and should be interpreted cautiously. FOI may provide an additional diagnostic method in pediatric rheumatology.
Trial registration: Deutsches Register Klinischer Studien DRKS00012572 . Registered 31 July 2017.
Keywords: Arthralgia; Fluorescence optical imaging; Imaging; Juvenile idiopathic arthritis; Power Doppler; Ultrasound.
Conflict of interest statement
Ethics approval and consent to participate
The study was performed in compliance with the Declaration of Helsinki. The study protocol was approved by the local ethics committee of the Charité University Medicine Berlin, Germany (No. EA2/126/12). All study participants were informed about the examination process, risks, and potential side effects of the fluorescence dye. Written informed consent was obtained from all participants.
Consent for publication
By signing informed consent, the participations in this study (patients and parents) approved the use of their pseudonymized data for scientific analysis.
Competing interests
MCB, A-MG, SO, RT, SGW, MB, GRB, TK, HG, and JK declare that they have no competing interests. KM has received honoraria for lectures from Pfizer, Roche, and Pharm-Allergan. GH has received grants and honorary fees from Abbvie, Pfizer, Novartis, and Roche/Chugai.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Fluorescence optical imaging: ready for prime time?RMD Open. 2021 Jun;7(2):e001497. doi: 10.1136/rmdopen-2020-001497. RMD Open. 2021. PMID: 34088778 Free PMC article. Review.
-
Fluorescence optical imaging and musculoskeletal ultrasonography in juvenile idiopathic polyarticular disease before and during antirheumatic treatment - a multicenter non-interventional diagnostic evaluation.Arthritis Res Ther. 2017 Jun 30;19(1):147. doi: 10.1186/s13075-017-1355-4. Arthritis Res Ther. 2017. PMID: 28666454 Free PMC article.
-
Near-infrared Fluorescence Optical Imaging in Early Rheumatoid Arthritis: A Comparison to Magnetic Resonance Imaging and Ultrasonography.J Rheumatol. 2015 Jul;42(7):1112-8. doi: 10.3899/jrheum.141244. Epub 2015 May 1. J Rheumatol. 2015. PMID: 25934821
-
Analysis of distribution and severity of inflammation in patients with osteoarthitis compared to rheumatoid arthritis by ICG-enhanced fluorescence optical imaging and musculoskeletal ultrasound: a pilot study.Ann Rheum Dis. 2016 Mar;75(3):566-70. doi: 10.1136/annrheumdis-2015-207345. Epub 2015 Aug 26. Ann Rheum Dis. 2016. PMID: 26311723 Free PMC article.
-
What is the value of musculoskeletal ultrasound in patients presenting with arthralgia to predict inflammatory arthritis development? A systematic literature review.Arthritis Res Ther. 2018 Oct 11;20(1):228. doi: 10.1186/s13075-018-1715-8. Arthritis Res Ther. 2018. PMID: 30305156 Free PMC article.
Cited by
-
An objective, automated and robust scoring using fluorescence optical imaging to evaluate changes in micro-vascularisation indicating early arthritis.PLoS One. 2022 Sep 27;17(9):e0274593. doi: 10.1371/journal.pone.0274593. eCollection 2022. PLoS One. 2022. PMID: 36166433 Free PMC article.
-
Fluorescence optical imaging: ready for prime time?RMD Open. 2021 Jun;7(2):e001497. doi: 10.1136/rmdopen-2020-001497. RMD Open. 2021. PMID: 34088778 Free PMC article. Review.
-
[Can rheuma be scanned? : Review of the current study situation on fluorescence optical imaging].Z Rheumatol. 2023 Oct;82(8):627-637. doi: 10.1007/s00393-023-01404-8. Epub 2023 Aug 25. Z Rheumatol. 2023. PMID: 37626223 Review. German.
-
Optical spectral transmission to assess glucocorticoid therapy response in patients with arthritis: a longitudinal follow-up comparison with joint ultrasound.Arthritis Res Ther. 2023 Mar 25;25(1):47. doi: 10.1186/s13075-023-03023-9. Arthritis Res Ther. 2023. PMID: 36964628 Free PMC article. Clinical Trial.
-
Evaluation of three scoring methods for Fluorescence Optical Imaging in erosive hand osteoarthritis and rheumatoid arthritis.Osteoarthr Cartil Open. 2019 Dec 9;1(3-4):100017. doi: 10.1016/j.ocarto.2019.100017. eCollection 2020 Jan-Feb. Osteoarthr Cartil Open. 2019. PMID: 36475004 Free PMC article.
References
-
- Haas J. Chronische muskuloskelettale Schmerzen bei Kindern und Jugendlichen. Monatsschr Kinderheilkd. 2009;157:647–54. doi: 10.1007/s00112-009-1959-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

